I. Introduction
According to World Health Organization, the current pandemic of coronavirus disease 2019 (COVID-19) caused about 200 million diagnosed cases and 40 million deaths worldwide by August 1, 20211. Patients with COVID-19 infection not only presents fever, respiratory symptoms, muscle or body aches2, but also appear nausea/vomiting, neurological and neuromuscular symptoms3,4. Due to the lack of knowledge about the virus itself and insufficient therapeutic agents, vaccination against COVID-19 virus is considered a key factor in combating to this disease5.
On the other hand, the number of people vaccinated increases rapidly, and then multiple neurological complications are reported following this vaccination including olfactory dysfunction (phantosmia or anosmia), acute encephalitis, myositis, Guillain-Barré syndrome (GBS)6,7. Regarding GBS, 227 cases per 51.4 million doses after vaccination were reported by June 27, 20218. Although underlying mechanisms are not clearly investigated, the possibility of causal relationship between COVID-19 vaccination and neurological complications has being raised9,10. However, there are not yet established treatments for the neurological adverse effects associated with COVID-19 vaccination. Furthermore, the complications of COVID-19 vaccination are even underestimated by the benefits of the vaccine11.
We herein present a clinical case of GBS-like neurological symptoms including limb weakness, phantosmia and nausea/vomiting after COVID-19 vaccination treated with traditional Korean medicine (TKM). This study followed the Case Report Guideline12 and was approved by the Institutional Review Board of Daejeon University Korean Medical Hospital (DJDSKH-21-E-33-1) with patient’s informed consent.
II. Case presentation
A 73-year old man was admitted to our hospital due to worsened limb weakness, phantosmia and excessive vomiting. As seen in Fig. 1A, he had received the first dose of ChAdOx1 nCov-19 (AstraZeneca) vaccine at June 7 th 2021. From 3 days after vaccination, he had experienced phantosmia and limb weakness, and the symptoms persisted for more than a month. The symptoms continued to worsen and he complained dizziness and nausea/vomiting 3 months after vaccination. The serial medical examinations including a gastroscopy and colonoscopy in July, and brain magnetic resonance imaging/ magnetic resonance angiography (MRI/MRA) and electromyography in August didn’t present any abnormal findings. In addition, no abnormalities were found in the otolaryngology examination at September.
Fig. 1
Summary of clinical courses and treatment outcomes.
(A) Clinical courses before admission in our hospital. (B) Treatment outcomes while admission.
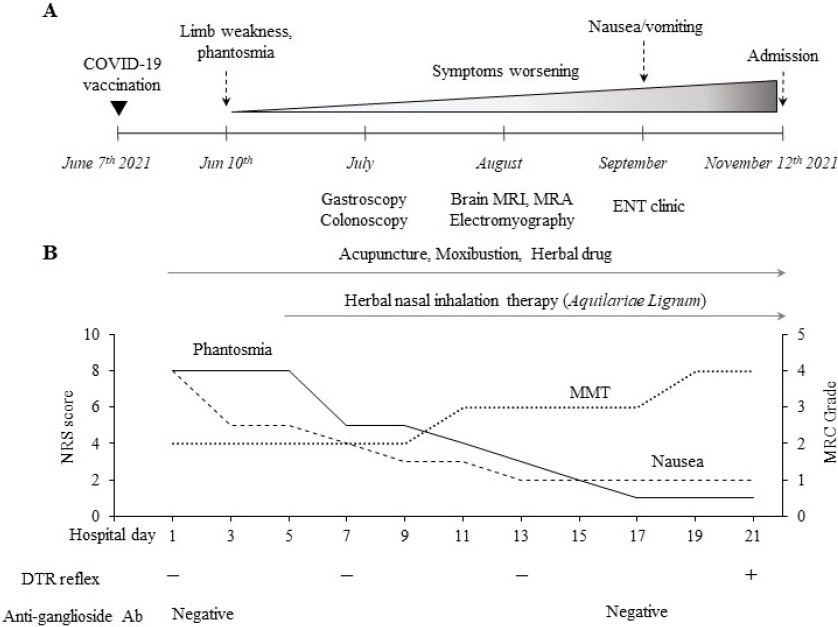
The patient had lost about 10.0 kg (from 73.0 to 63.0 kg) of weight during past 4 months, leading to thin as a 20.1 of body mass index (BMI), respectively. He was unable to walk on his own due to worsening malaise and particularly weakness of lower extremities. The patient and his family frustrated, which led to choose TKM and visit our hospital on October 20 th 2021. He had to use a wheelchair on arrival, and the vital signs on arrival were stable, except a mild fever (37.1 °C) and a high blood pressure (174/95 mmHg for systolic/diastolic). He had medical history of glaucoma and cataract diagnosed at 2 year-ago. He doesn’t take any medication. He was an ex-smoker, drank socially, and drank coffee (3 cups/day). He had worked as rancher until just this episode happening, and he has a family history of liver cancer.
The neurological examination at presentation revealed loss of deep tendon reflexes (DTR) at four limbs. In particular, both lower limb weakness was severe as grade 2 (grading 0 to 5 scale) according to Medical Research Council (MRC) manual muscle test. The result assessed by MRC manual muscle test can reveal information about neurologic deficits13. The mental state was alert, pupil reflexes were both good, and neck stiffness, Babinski reflex and Hoffmann sign were all negative. But, the absence of ankle clonus was existed.
Laboratory tests showed no abnormality except for the increased erythrocyte sedimentation rate (ESR, 46 mm/hr) and decreased serum sodium level (130 mmol/L). A slight hyperthyroidism pattern was observed in blood test, evidenced by a slight low level of thyroid stimulating hormone (TSH, 0.08 uIU/mL) and slight high free T4 level (1.85 ng/dL). On 12 days after admission, the follow-up laboratory tests showed normal ranged results including free T4 level (1.54 ng/dL) and ESR (28 mm/hr). TSH level also increased, but it was slightly lower than the normal range (0.13 uIU/mL). To check the possibility of GBS, we conducted anti-ganglioside IgG/IgM antibody (anti GM1, FD1b, GQ1b IgG/IgM isotype) tests on day 1 and day 17 and the results were negative. Despite the incompatible findings, we thought that there might be an association between vaccination and neurological symptoms, which suspected as GBS.
We performed acupuncture treatment twice daily 20 minutes at mainly 6 acupoints (CV23 (Lianquan), LI4 (He Gu), LI20 (Yingxiang), LR3 (Taichong), PC6 (Neiguan), and ST36 (Zusanli), as seen in Fig. 2) using sterile acupuncture needles (diameter: 0.20 mm, length: 15 mm, Dong Bang Acupuncture Co., Korea). Moxibustion treatment was also given on three acupoints (Fig. 2), CV4 (Guanyuan), CV8 (Shenque), CV12 (Zhongwan), with orally administration of an herbal medicine, Banhabakchulchunma-tang (as a 70 mL decoction 3 time daily, Table 1). In addition, we conducted a nasal inhalation therapy of Aquilariae Lignum, dissolved 1~2 g in 500 mL of distilled boiling water, and then inhaled for 30 minutes every day. Also, he was treated with IV metoclopramide 10 mg for 5 days and total parenteral nutrition for 6 days.
Fig. 2
Images showing the treatment point and picture of medical devices.
(A) Acupoints of acupuncture (blue) and moxibustion (red) treatment. (B) Pictures of electronic moxibustion for CV8 (left) and CV4, CV12 (right). (C) Picture of nasal inhalation treatment.
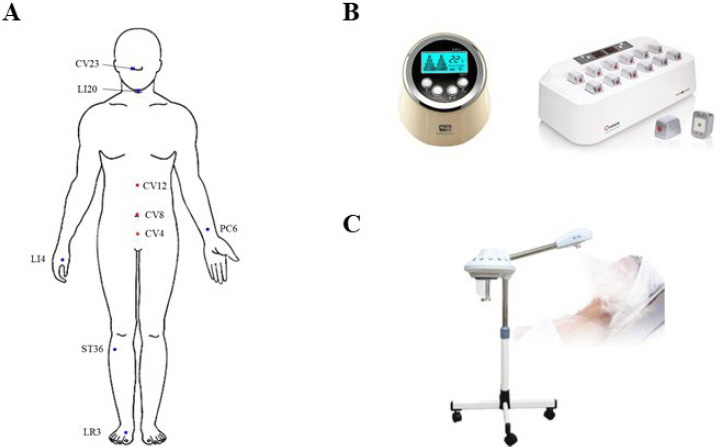
Table 1
Composition of Banhabakchulchunma-tang
| Scientific name | Herbal name | Daily dose* |
|---|---|---|
| Pinellia ternata (Thunb.) Makino | Pinelliae Tuber | 36 g |
| Citrus×aurantium L. | Citri Unshius Pericarpium | 36 g |
| Hordeum vulgare L. | Hordei Fructus Germinatus | 36 g |
| Atractylodes lancea (Thunb.) DC. | Atractylodis Rhizoma Alba | 24 g |
| - | Massa Medicata Fermentata | 24 g |
| Atractylodes lancea (Thunb.) DC. | Atractylodis Rhizoma | 12 g |
| Poria cocos Wolf | Poria Sclerotium | 12 g |
| Panax ginseng C.A.Mey. | Ginseng Radix | 12 g |
| Astragalus mongholicus Bunge | Astragali Radix | 12 g |
| Gastrodia elata Blume | Gastrodiae Rhizoma | 12 g |
| Alisma plantago-aquatica subsp. orientale (Sam.) Sam. | Alismatis Rhizoma | 12g |
| Zingiber officinale Roscoe | Zingiberis Rhizoma | 6 g |
| Phellodendron amurense Rupr. | Phellodendri Cortex | 6 g |
|
|
||
| Total | 120 g | |
As summarized in Fig. 1B, the phantosmia and nausea/vomiting were mostly relieved from 8 to 5 and 4 on the day 7 of admission by self-reporting numerical rating scale (NRS). The neurological symptoms and muscle strength of both lower limbs was also gradually recovered. On day of 17 after admission, including a weight gain of 4.0 kg (63.0 to 67.0 kg), patient was able to ambulate without walker and MRC grade was 3/5 throughout the both lower limbs. After 22 days on hospitalization, he discharged with ambulating on his own with an MRC grade of 4/5 on both lower limbs.
III. Discussion
We herein reported a case of 73-year old man who presented neurological symptoms including limb weakness, phantosmia and nausea/vomiting after receiving his first dose of COVID-19 vaccination.
Olfactory dysfunction including phantosmia and anosmia is considered as important symptom in patients infected with COVID-193. Several case reports published olfactory hallucinations after COVID-19 infection14,15. In addition, there is a case series of 6 patients reporting olfactory disorder after COVID-19 vaccination with AstraZeneca (n=4) and Pfizer (n=2)16. One study reported that a patient with phantosmia revealed the olfactory bulbs and tracts enhancement in brain MRI after administration of COVID-19 vaccine16.
On the other hand, GBS is the most common autoimmune disorder of the peripheral nervous system, with an incidence of 1.1/100,000 worldwide17. GBS often occurs in patients with a history of infection and is accompanied by symptoms of preceding infections18. Notably, various case of GBS have been reported following vaccination19-21. Although anti-ganglioside IgG/IgM antibodies were negative in our case, the patient showed clinically similar symptoms with GBS. Antiganglioside antibodies have a sensitivity of only 36% in GBS patients22. According to one study, of 35 patients associated with COVID-19 related GBS, anti-GD1b and anti-GM1 antibodies were positive in only two cases, while 33 patients were negative23. Although electrophysiological examination is of significance for diagnosis of GBS, the normal finding in electromyography can be often shown especially in early case of GBS24. His electromyography was negative when he was examined on approximately 2 months after vaccination (2 months before visiting our hospital) in another clinic. There are some studies focusing on long-term neurological problem following COVID-19, which suggests the possibility of delayed neurological symptoms25,26. The optimal treatment for rapidly progression GBS is believed to receive an intravenous immunoglobulin or plasma exchange, but supportive care is adequate treatment option for mild to moderate GBS patients27. In this patient case, the previously visited doctors couldn’t make a clear diagnosis of disease due to any pathologic finding in objective examinations, and furthermore his symptoms didn’t respond to conventional treatments.
We conducted TKM-based treatments including acupuncture, moxibustion and herbal drug. Acupuncture and moxibustion treatment are effective in treating nausea and vomiting28,29. Moreover, several studies have reported cases that post-viral olfactory disorder improved after acupuncture treatment30,31. Banhabakchulchunma-tang, a traditional herbal formula composed of 60 g of 14 herbs (Table 1), is usually prescribed to treat gastrointestinal diseases with neurological symptoms32,33. One study has shown that BCT has a protective effect against neuronal cell damage in brain and increases the expression of acetylcholine esterase in the hippocampus34. Several individual herbs composing Banhabakchulchunma-tang have shown anti- inflammatory, antioxidant, gastro-protective effects35-37. We also administered the Aquilariae Lignum through intranasal inhalation. Aquilariae Lignum has been traditionally used for inflammation and relieving neurological symptoms, and then we previously reported its neuroprotective effects via modulation of neuroexcitotoxicity and neuroinflammation38,39.
In summary, the present case showed the GBS-like neurological complications after COVID-19 vaccination, and the TKM-based treatment. Our study however had limitations including the lack of evience for diagnosis and the possibility of controversial causal relationship between symptoms and COVID-19 vaccination. Considering the short-term improvements with treatment and the intervention of psychological factors, GBS cannot be definitively diagnosed. Our study provides the potential of TKM-based therapies to manage especially COVID-19 vaccination- and hopefully infection-related complications. Further clinical studies are required to apply the interventions for patients suffering from long-term neurological complains related COVID-19 vaccine in the future.











