위암 수술 후 장마비에 대한 한의학적 치료의 효과 : 체계적 문헌 고찰 및 메타분석
Abstract
Objectives:
Postoperative ileus (POI) is a common impairment of gastrointestinal motility and causes a delay in postoperative recovery, as well as an increased length of hospital stay, but no single strategy has a significant recuperative effect on POI. Studies of traditional Korean medicine (TKM) have reported improvements in bowel function after surgery. The aim of this systematic review was to assess the effectiveness of TKM on postoperative ileus in gastric cancer patients.
Methods:
We used six databases to search for studies published from January 1, 2007, until May 11, 2022. The included studies were those reporting gastric cancer patients who received TKM treatment after gastrectomy through indicators related to POI.
Results:
The search identified 27 RCTs that used herbal medicine (Daegeonjung-tang and Gami-leejoongtang), herbal medicine combined with acupuncture (Sama-tang, Gumiseunggi-tang, Daeseunggi-tang, and Insam-tang), acupuncture, acupuncture and moxibustion, electroacupuncture, warm needling, transcutaneous electroacupuncture (TEA), low-frequency electrical acupoint stimulation (LEAS), moxibustion, auricular acupressure, and ST-36 acupoint injection with neostigmine as treatments for POI. The time to first flatus was shortened by herbal medicine combined with warm needling (Sama-tang, Gumiseunggi-tang, Daeseunggi-tang, and Insam-tang), acupuncture, electroacupuncture, warm needling, TEA, moxibustion, auricular acupressure, and ST-36 acupoint injection with neostigmine (p<0.00001). The time to the first defecation decreased significantly in response to the herbal medicine combined with warm needling (Sama-tang, Gumiseunggi-tang, Daeseunggi-tang, and Insam-tang), acupuncture, electroacupuncture, warm needling, TEA, moxibustion, auricular acupressure, and ST-36 acupoint injection with neostigmine (p<0.00001). No serious adverse events occurred.
Conclusions:
TKM could be a promising option for preventing and resolving POI in gastric cancer patients after gastrectomy.
Keywords: postoperative ileus, gastric cancer, gastrectomy, traditional Korean medicine, meta-analysis
초 록
목적:
본 연구는 위암 수술 후 장마비의 한의학적 치료 효과를 평가하기 위해 수행하였다.
방법:
2007년 1월 1일부터 2022년 5월 11일까지 출판된 연구들을 6개의 데이터베이스를 통해 수집하였다. 수술 후 한의학적 치료를 받은 위암 환자를 장마비 관련 지표들을 통해 관찰한 연구들을 선정하였다.
결과:
한약, 침, 뜸, 전기혈위자극, 이혈요법, 족삼리혈의 약물 주입을 수술 후 장마비 치료법으로 사용한 27편의 연구를 선정하였다. 한약, 한약과 침 병용요법, 침, 뜸, 전기혈위자극, 이혈요법, 족삼리혈의 네오스티그민 주입 치료군에서 수술 후 첫 가스 배출까지의 시간이 감소하였고 (p<0.00001), 수술 후 첫 배변까지의 시간이 한약. 한약과 침 병용요법, 침, 뜸, 전기혈위자극, 이혈요법, 족삼리혈의 네오스티그민 주입 치료군에서 유의미하게 감소하였다 (p<0.00001). 심각한 이상반응은 나타나지 않았다.
결론:
한의학적 치료는 위절제술 후 위암 환자에게 수술 후 장마비의 예방과 치료법으로 활용될 수 있으며 수술 후 장마비 치료의 임상적 효과를 명확히 하기 위해 후속 연구가 필요하다.
중심어: 수술 후 장마비, 위암, 위절제술, 한의학적 치료, 메타분석
I. Introduction
Gastric cancer is the fifth most common cancer and the fourth leading cause of cancer deaths worldwide in 2020 1. The most recommended treatment is radical gastrectomy with lymph node dissection for patients with resectable early gastric cancer, as patients have improved survival after complete resection. Nevertheless, patients typically have a relatively slow postoperative recovery after gastrectomy, resulting in decreased survival and an increased length of hospital stay 2-4. Postoperative ileus (POI) is transient impairment of gastrointestinal motility lasting 3-5 days after surgery. It is characterized by nausea, vomiting, bloating, abdominal pain, loss of bowel movement, and the inability to pass flatus 5. When this dysfunction lasts beyond the expected time, it is called “paralytic” or “prolonged” postoperative ileus (PPOI) 6,7. Preventing POI is important to shorten the postoperative recovery period after gastrectomy 8 because PPOI is one of the main factors for a delay in postoperative recovery 9,10. The most commonly used clinical criteria to assess the resolution of POI are passage of flatus and stool, tolerance of an oral diet, and the presence of bowel sounds, so the primary therapeutic target in patients with POI is to shorten the time to first flatus and defecation 7. As POI is a major obstacle to rapid recovery after abdominal surgery, including gastrectomy, pharmacological and nonpharmacological treatments have been attempted, but have been proven to be either ineffective or minimally effective 11,12. Traditional Korean medicine (TKM) treatment is becoming a promising option to treat POI. TKM treatments, including acupuncture, acupressure, and herbal medicines, have a positive effect on the recovery of bowel function in cancer patients 13-15. However, comprehensive research on the effect of TKM treatment on POI in gastric cancer patients has not been conducted. Therefore, we conducted a systematic review and meta-analysis to assess the effectiveness of TKM in improving bowel function and preventing or treating POI in gastric cancer patients.
II. Methods
1. Inclusion and exclusion criteria
1) Types of study
We included all prospective randomized clinical trials (RCTs) available across the six databases (PubMed, the Cochrane Library, RISS, KISS, CNKI, and J-STAGE) selected. We excluded case studies, case series, and retrospective clinical studies.
2) Types of participant
We included gastric cancer patients after surgery.
3) Types of intervention
We included studies using Traditional Korean Medicine (herbal medicine, moxibustion, acupuncture, electro-acupuncture), regardless of the combination of Western medicine. The inclusion criteria for the controls were no treatment, placebos, or conventional medicines.
4) Types of outcome measure
The data of studies could be pooled for time to first flatus (twenty-six studies), time to the first defecation (twenty-one studies), the incidence of postoperative ileus (three studies), and adverse events (nine studies).
(1) Primary outcomes: time to first flatus, time to the first defecation
(2) Secondary outcomes: incidence of postoperative ileus, adverse events
2. Search methods
1) Searched electronic databases
We used six databases to search for limited languages in English, Japanese, Chinese, and Korean among the studies published from Jan 1, 2007, until May 11, 2022. The databases included were PubMed, the Cochrane Library, two Korean medical databases (the Research Information Service System[RISS], the Korean Studies Information Service System[KISS]), one Chinese medical database (China National Knowledge Infrastructure[CNKI]), and one Japanese medical database(Japan Science Technology Information Aggregator, Electronic [J-STAGE]).
2) Search strategy
The search terms we used were ‘gastric cancer’ OR ‘gastric neoplasm’ OR ‘gastric adenocarcinoma’ OR ‘stomach cancer’ OR ‘stomach neoplasm’ OR ‘stomach neoplasms’ OR ‘gastrectomy’ AND postoperative OR postsurgical OR ileus OR gastroparesis OR ‘gastrointestinal function’ OR ‘gastrointestinal motility function’ OR surgery AND ‘herbal medicine’ OR ‘Korean medicine’ OR ‘Chinese medicine’ OR moxibustion OR acupuncture OR herb OR plant OR ‘auricular pressure’ OR ‘auricular acupressure’. The search strategy involved using a mixture of the search terms in Korean and Chinese.
3. Selection of studies and data extraction
Two reviewers (HRB, EJK) selected potentially eligible articles by reading through titles and abstracts. These reviewers examined hard copies of the publications to determine their suitability. Any disagreements were resolved through discussion, and where necessary, in consultation with a third reviewer (NHL).
Two reviewers (HRB, EJK) extracted data from the studies included. Information that was excluded was as follows; the characteristics of subjects, the outcomes, and the results. We resolved disagreements through discussion and in consultation with the third author (NHL), who acted as an arbiter.
4. Assessment of risk of bias
Two reviewers (HRB, EJK) independently evaluated the risk of bias (ROB) in the studies included by following the guidelines given in the “Cochrane Handbook of Systematic Reviews of Interventions (i.e., sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting)”.
5. Data synthesis
We presented dichotomous data in terms of risk ratios, with 95% confidence intervals (CIs). The mean difference with 95% CIs for continuous data is used in this study.
The Review Manager 5.4.1, the Cochrane Collaboration’s software program, was used to perform the statistical analysis. We used weighted mean differences (WMD) with 95% confidence intervals (CIs) for calculating continuous data, and I2 test for assessing the heterogeneity of the data. If heterogeneity existed (I2≥50%), a random-effects model was applied. If or not, a fixed-effects model was applied. Statistically significant difference was considered as p<0.05.
III. Results
1. Study description
Of 76 eligible study hits, twenty-six studies were excluded after duplicates were removed. After assessing the fifty abstracts of the studies, twenty studies are excluded. After assessing the thirty full-text articles, three studies were excluded for various reasons ( Fig. 1). Finally, twenty-seven studies met our inclusion criteria 16-42. Summaries of the key data from these studies are shown in Table 1. Of the twenty-seven studies, one trial was conducted in Korea 19, two were performed in Japan 18,20, and twenty-four were conducted in China 16,17,21-42. Twenty-five studies included gastric cancer patients after gastrectomy (laparoscopic gastrectomy 29,37, total gastrectomy 20,24, open total gastrectomy 18, distal gastrectomy 19,42, or total or partial gastrectomy 17,21-23,25-27,30-36,38-40,41), one study was undertaken with gastric cancer patients diagnosed with PPOI following gastrectomy 16, and the other one study was conducted for abdominal distension patients after gastrectomy 28. Test interventions included Daegeonjung-tang (Daikenchuto; DKT, Da-Jian-Zhong-Tang) (two studies), Gami-leejoongtang (modified Lizhong tang) (one study), Sama-tang (Simotang) combined with warm needling (two studies), Gumiseunggi-tang (Jiuwei Chengqi Oral Liquid) combined with warm needling (one study), Daeseunggi-tang (Dachengqi decoction) combined with warm needling (one study), Insam-tang (Renshen decoction) combined with acupuncture (one study), acupuncture (three studies), acupuncture and moxibustion (one study), warm needling (two studies), electroacupuncture (one study), transcutaneous electroacupuncture (TEA) (one study), transcutaneous electrical acupoint stimulation (TEAS) (one study), low-frequency electrical acupoint stimulation (LEAS) (one study), acupoint injection with neostigmine (one study), and moxibustion (four studies), and auricular acupressure (four studies).
Fig. 1
Flow chart of study selection process. 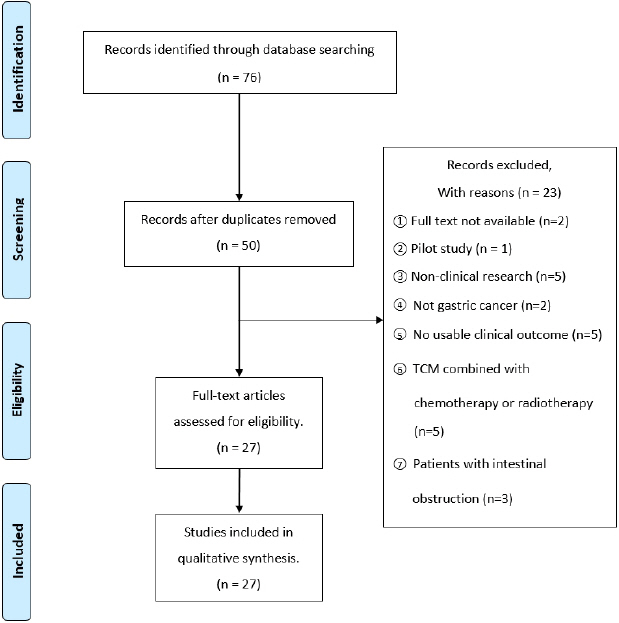
Table 1
Summary of included Randomized Clinical Trials
|
First Author (year) /nation |
Sample size and condition |
Intervention |
Control intervention |
Main outcome measures |
Results (P<0.05) |
Adverse effects |
|
Kai-Bo Chen (2018) /China |
63 patients, total or partial gastrectomy |
(A) TEA (2x/d, 1 h, from
POD 1 until passing flatus or POD 5, n=33) (Acupoint : ST-36, PC-6) |
(B) Non-TEA (n=30) |
(1) time to first flatus
(2) bowel movement
(3) time to nasogastric tube removal
(4) incidence of prolonged ileus |
(1) (A)<(B) (P=0.038)
(2) bowel sound on POD 2 (/min) : (A)>(B) (P=0.017)
(3) (A)<(B) (P=0.049)
(4) NS : (A)=0, (B)=3 (P=0.102) |
No serious AE |
|
|
Kozo Yoshikawa (2015) /Japan |
195 patients, open total gastrectomy |
(A) DKT (3x/d, total 15.0 g/d, POD 1~12, n=96) |
(B) Placebo (n=99) |
(1) time to first flatus
(2) time to first defecation
(3) incidence of postoperative ileus |
(1) NS : (A)>(B) (P=0.95)
(2) (A)<(B) (P=0.05)
(3) NS : (A)=3, (B)=2 |
Diarrhea (2),
Anorexia (1),
Hypoalbuminemia (1), Nausea (1),
Elevated amylase level (1)
No serious AE |
|
|
Se Yun Jung (2017) /Korea |
36 patients, distal gastrectomy |
(A) Acupuncture (1x/d, 20 mm depth, 25~30 min,
POD 1~5, n=18) (Acupoint : bilateral
ST-36, SP-6, LI-4, TE-6 (Electrical stimulation, 100 Hz) LV-3, LI-11 ; unilateral GV-20,
EX-HN3, GV-26, CV-24) |
(B) Non-acupuncture (n=18) |
(1) number of remnant sitz markers in the small intestine
(2) time to first flatus
(3) time to first defecation |
(1) at day 3 :
(A)<(B) (P<0.001); day 5 :
(A)<(B) (P<0.001) ; day 7 : NS (P=0.317)
(2) (A)<(B) (P=0.009)
(3) NS ; (A)<(B) (P=0.054) |
None |
|
|
Xiaolan You (2018) /China |
246 patients,
PPOI following gastrectomy |
(A) ST-36 acupoint inj. with neostigmine (1x/d, total of 1.0 mg, 0.5 mg per side until recovery of peristalsis, n=67) |
(B) Gluteal intramuscular inj. with 1.0 mg neostigmine (1x/d, n=67)
(C) ST36 acupuncture alone (1x/d, depth of 1-2 cun, 30 min, n=59)
(D) Standard therapy (n=53) |
(1) effectiveness rate for recovery of peristalsis
(2) time to first flatus
(3) time to first defecation
(4) time to bowel sound recovery |
(1) passed flatus or defecated within two hours following treatment : (A)>(B)>(D) (P<0.01, P<0.01, P<0.01)
(2) (A)<(B)<(D) (P<0.01, P<0.01, P<0.01)
(3) (A)<(B)<(D) (P<0.01, P<0.01, P<0.01)
(4) the time of bowel sound recovery (more than 3x/min) :
(A)<(B)<(D) (P<0.01, P<0.01, P<0.01) |
Abdominal pain score, rate of diarrhea, nausea, vomiting, tearing, delirium, anxiety :
(A)<(B) (P<0.05) |
|
|
Yusuke Akamaru (2015) / Japan |
81 patients, total gastrectomy |
(A) DKT (3x/d, total 7.5 g/d for 3 months starting at POD 1, n=41) |
(B) 20 ml tepid water (3x/day, n=40) |
(1) time to first bowel movement
(2) stool attributes
(3) the quantity of bowel gas
(4) incidence of postoperative ileus |
(1) NS (P=0.811)
(2) number of stools per day :
(A)>(B) (P=0.037) ;
Bristol stool scale :
(A)>(B) (P=0.041)
(3) GVS at 7 days :
(A)<(B) (P<0.05) ; at 1 month :
(A)<(B) (P<0.05) ; at 3 months :
(A)<(B) (P<0.05) (4) NS : (A)=1, (B)=2 |
1.9% of (A) : gastrointestinal or hepatobiliary disorders
No serious AE |
|
|
Xin Zhou (2021) /China |
81 patients, standard laparoscopic or robot radical gastrectomy |
(A) TEAS (2x/d, 30 min,
POD 1~3, n=41) (Acupoint : LI-4, PC-6,
BL-21, BL-27, ST-36, ST-37) |
(B) Non-TEAS (n=40) |
(1) time to first flatus
(2) time to first defecation |
(1) (A)<(B) (P<0.001)
(2) (A)<(B) (P<0.001) |
None |
|
|
He Dan (2020) /China |
177 patients, gastrectomy |
(A) LEAS (1x/d, 30 min, from POD 1 to first operative flatus, n=45) (Acupoint : ST-36, ST-37, ST-39, SP-6) |
(B) Non-LEAS (n=43)
(C) FTS (n=46)
(D) FTS+LEAS (n=43) |
(1) time to first flatus
(2) time to first defecation |
(1) (A)<(B) (P<0.05) ;
(D)<(C)<(B) (P<0.05, P<0.05, P<0.05) (2) (A), (C), (D)<(B) (P<0.05, P<0.05, P<0.05) |
None |
|
|
Chen Qian Qian (2018) /China |
90 patients, gastrectomy |
(A) Simotang (3x/d, total 60 ml/d, POD 1~10, n=30)
(B) Simotang (3x/d, total 60 ml/d, POD 1~10) +Warm needling (POD 3~10, n=30) (Acupoint : CV-12, ST-25, ST-36, ST-37, ST-39, LR-3, PC-6, LI-4, SP-6) |
(C) Standard therapy (n=30) |
(1) time to first flatus
(2) time to first defecation |
(1) (B)<(A)<(C) (P<0.05, P<0.05, P<0.05)
(2) (B)<(A)<(C) (P<0.05, P<0.05, P<0.05) |
Not stated |
|
|
Cheng Caihong (2019) /China |
80 middle aged and elderly patients, total gastrectomy |
(A) Jiuwei Chengqi oral liquid (3x/d, total 60 ml/d, 2 wk) +Warm needling (1x/d, 30 min, 2 wk, n=40) (Acupoint : PC-6,
ST-36, LR-3, CV-6, SP-6) |
(B) Jiuwei Chengqi oral liquid (3x/d, total 60 ml/d, 2 wk, n=40) |
(1) time to first flatus
(2) time to first defecation |
(1) (A)<(B) (P<0.05)
(2) (A)<(B) (P<0.05) |
Not stated |
|
|
Huang Wei Jun (2014) /China |
200 patients, gastrectomy |
(A) Auricular acupressure (q6hrs, 30~60 sec, 1 wk, n=100) (Acupoint: Shenmen, Sympathetic, Subcortex, LI, SI, Stomach, Spleen) |
(B) Standard therapy (n=100) |
(1) time to bowel sound appearance
(2) time to first flatus
(3) time to first defecation |
(1) (A)<(B) (P<0.001)
(2) (A)<(B) (P<0.001)
(3) (A)<(B) (P=0.032) |
None |
|
|
Nan Nan (2018) /China |
70 patients, gastrectomy |
(A) Auricular acupressure (5x/d, 1~2 min, n=35) (Acupoint : SI, Skin, Sympathetic, Stomach, Shenmen, LI) |
(B) Standard therapy (n=35) |
(1) time to first flatus
(2) time to first defecation |
(1) (A)<(B) (P<0.05)
(2) (A)<(B) (P<0.05) |
Not stated |
|
|
Qian Chan Lin (2017) /China |
60 patients, gastrectomy |
(A) Acupuncture (1x/d, 20 min, POD 1~7, n=30) (Acupoint : ST-36, ST-37, ST-39) |
(B) Standard therapy (n=30) |
(1) time to bowel sound appearance
(2) time to first flatus |
(1) (A)<(B) (P<0.01)
(2) (A)<(B) (P<0.01) |
Not stated |
|
|
Ren Dan (2016) /China |
64 patients, abdominal distension after gastrectomy |
(A) Moxibustion (1x/d, 1 wk, n=32) (Acupoint : ST-36, CV-8) |
(B) Standard therapy (n=32) |
(1) time to bowel sound appearance
(2) time to first flatus |
(1) (A)<(B) (P<0.01)
(2) (A)<(B) (P<0.01) |
Not stated |
|
|
Tan Ping (2017) /China |
68 patients (over the age of 60 years), laparoscopic gastrectomy |
(A) Auricular acupressure (q2hrs, 0.5~1 min, from POD 1 until bowel sound recovery, n=34) (Acupoint : Stomach, SI, LI, Sanjiao, Kidney) |
(B) Standard therapy (n=34) |
(1) time to bowel sound appearance
(2) time to first flatus
(3) time to first defecation |
(1) (A)<(B) (P=0.001)
(2) (A)<(B) (P=0.041)
(3) NS ;
(A)<(B) (P=0.726) |
Not stated |
|
|
Wang Ai Jun (2018) /China |
28 patients, gastrectomy |
(A) Warm needling (n=14) (Acupoint : ST-36, PC-6, SP-6, LR-3, KI-3, LI-11) |
(B) Standard therapy (n=14) |
(1) time to first flatus
(2) time to first defecation
(3) time to bowel sound appearance |
(1) (A)<(B) (P<0.01)
(2) (A)<(B) (P=0.002)
(3) (A)<(B) (P=0.013) |
Not stated |
|
|
Xu rui (2017) /China |
162 patients, gastrectomy |
(A) Moxibustion (1x/d, 20 min, from POD 1 until passing flatus or POD 6, n=55) (Acupoint : ST-36)
(B) Ligitongfufang Footbath (1x/d, 30 min, from POD 1 until passing flatus or POD 6, n=53) |
(C) Standard therapy (n=54) |
(1) time to bowel sound appearance
(2) time to first flatus
(3) time to first defecation |
(1) (A), (B)<(C) (P<0.05, P<0.05)
(2) (A), (B)<(C) (P<0.05, P<0.05)
(3) (A), (B)<(C) (P<0.01, P<0.05) |
Not stated |
|
|
Ye Guandi (2018) /China |
175 patients, gastrectomy |
(A) Auricular acupressure (q6hrs, 0.5~1 min, 1 wk, n=88) (Acupoint : Sympathetic, Shenmen, Subcortex, LI, SI, Spleen, Stomach) |
(B) Standard therapy (n=87) |
(1) time to bowel sound appearance
(2) time to first flatus
(3) time to first defecation |
(1) (A)<(B) (P<0.05)
(2) (A)<(B) (P<0.05)
(3) (A)<(B) (P<0.05) |
Not stated |
|
Yin Shuang
Hong (2009) /China |
90 patients, gastrectomy |
(A) Simotang (3x/d, total 60 ml/d, POD 1~10, n=30)
(B) Simotang (3x/d, total 60 ml/d, POD 1~10) +Warm needling (1x/d, 45 min, POD 1~7, n=30) (Acupoint : ST-36, PC-6, SP-6, LR-3) |
(C) Standard therapy (n=30) |
(1) time to first flatus
(2) time to first defecation |
(1) (B)<(A), (C) (P<0.05, P<0.01) ;
(A)<(C) (P<0.05)
(2) (B)<(A), (C) (P<0.05, P<0.01) ; (A)<(C) (P<0.05) |
Not stated |
|
|
Zhang Ying Wei (2014) /China |
80 patients, gastrectomy |
(A) Moxibustion (1x/d, 10~15 min, from 6 h after surgery to 3d after passing flatus, n=40) (Acupoint : ST-36, ST-37, LI-4) |
(B) Standard therapy (n=40) |
(1) time to bowel sound appearance
(2) time to first flatus |
(1) (A)<(B) (P<0.01)
(2) (A)<(B) (P<0.01) |
Not stated |
|
|
Ynag Le (2022) /China |
80 patients, gastrectomy |
(A) Acupuncture (1x/d, 30 min, 10 d, n=40) (Acupoint : ST-36, SP-9, SP-6, ST-37, ST-39) |
(B) Standard therapy (n=40) |
(1) time to first flatus
(2) time to first defecation |
(1) (A)<(B) (P<0.05)
(2) (A)<(B) (P<0.05) |
Not stated |
|
|
Zhang Lan Jin (2021) /China |
76 patients, gastrectomy |
(A) Moxibustion (1x/d, 30 min (7a.m.~9a.m.), n=38) (Acupoint : CV-8, ST-25, CV-4, ST-36) |
(B) Standard therapy (n=38) |
(1) time to bowel sound appearance
(2) time to first flatus
(3) time to first defecation |
(1) (A)<(B) (P<0.05)
(2) (A)<(B) (P<0.05)
(3) (A)<(B) (P<0.05) |
Not stated |
|
|
Kang Wen Zhe (2021) /China |
162 patients, laparoscopic gastrectomy |
(A) Acupuncture (1x/d, POD 1~, n=54) (Acupoint : ST-36, SP-6, PC-6)
(B) Moxibustion (1x/d, 30 min, POD 1~, n=54) |
(C) Standard therapy (n=54) |
(1) time to first flatus |
(1) (A)<(C) (P<0.01)
(B)<(C) (P<0.01)
(A) : 59.41 h,
(B) : 59.09 h,
(C) : 64.72 h |
Not stated |
|
|
Li Xi Sheng (2021) /China |
60 patients, gastrectomy |
(A) Dachengqi decoction (1x/d, total 200 ml/d) +Warm needling (1x/d, 30~45 min, 7 days, n=30) (Acupoint : ST-36,
LI-4, PC-6) |
(B) Warm needling (1x/d, 30~45 min, 7 days, n=30) (Acupoint : ST-36,
LI-4, PC-6) |
(1) time to first flatus
(2) time to first defecation |
(1) (A)<(B) (P<0.05)
(2) (A)<(B) (P<0.05) |
Not stated |
|
|
Guo Jia Huan (2021) /China |
68 patients, gastrectomy |
(A) Acupuncture (1x/d, 30 min, POD 6~(1 mo)) (Acupoint : ST-36, LU-5) +Renshen decoction (2x/d, total 200 ml/d, POD 5~(1 mo), n=34) |
(B) Standard therapy (n=34) |
(1) time to bowel sound appearance (2) time to first flatus
(3) time to first defecation |
(1) (A)<(B) (P<0.05)
(2) (A)<(B) (P<0.05)
(3) (A)<(B) (P<0.05) |
Not stated |
|
|
Wu Jing (2021) /China |
98 patients, gastrectomy |
(A) Warm needling (7 d) (Acupoint : ST-36, PC-6, SP-6, LR-3, LI-4) +Acupoint massage (2x/d, 15~20 min, 7d, n=39) (Acupoint : PC-6, ST-36, LI-4) |
(B) Standard therapy (n=49) |
(1) time to bowel sound appearance (2) time to first flatus
(3) time to first defecation |
(1) (A)<(B) (P<0.01)
(2) (A)<(B) (P<0.01)
(3) (A)<(B) (P<0.01) |
Not stated |
|
|
Huang Yi Bo (2022) /China |
83 patients, gastrectomy |
(A) modified Lizhong tang (2x/d, total 400 ml/d, n=43) |
(B) Standard therapy (n=40) |
(1) time to bowel sound appearance (2) time to first flatus
(3) time to first defecation |
(1) (A)<(B) (P<0.05)
(2) (A)<(B) (P<0.05)
(3) (A)<(B) (P<0.05) |
Not stated |
|
|
Ding Yun Qing (2021) /China |
60 patients, distal gastrectomy |
(A) Electroacupuncture (1x/d, 20 min, 1d before Op, POD 1~5, n=30) (Acupoint : GV-20, PC-6, ST-25, ST-36) |
(B) Sham treaetment (20 min, POD 1~5, n=30) |
(1) time to first flatus
(2) time to first defecation |
(1) (A)<(B) (P<0.01)
(2) (A)<(B) (P<0.01) |
No serious AE |
2. Risk of bias
1) Random sequence generation
Sixteen studies reported using a random sequence generation method, and we rated random sequence generation as a low risk of bias 16,17,21,22,25-27,29,32,34,35,38,39,40-42. We rated the other eleven studies as having an unclear risk of bias because no randomization methods were described.
2) Allocation concealment
Allocation concealment was not described in any of the studies, and we rated the risk of bias as unclear.
3) Blinding of participants and personnel
Only two studies conducted double-blind trials by comparing the treatment groups with placebo groups; we rated these studies as having a low risk of bias 18,42. In the five trials, because the participants were not blinded, we rated them as having a high risk of performance bias 16,17,19-21. One of these studies reported that no blinding was applied to participants or physicians because the treatments had different characteristics 16. In the remaining trials, we rated them as having an unclear risk of performance bias because there was no statement about the blinding of participants.
4) Blinding of outcome assessment
The method for blinding outcome assessors was not reported for twenty-six trials, so we rated them as having an unclear risk of detection bias. We rated one study that reported outcome assessor blinding as having a low risk of detection bias 16.
5) Incomplete outcome data
Four studies reported the reasons for discontinuing the intervention, and we rated these as having a low risk of bias 16,18-20. The other studies had an unclear risk of attrition bias because they did not report dropout cases.
6) Selective reporting
One study predefined the outcomes and demonstrated all predefined measurements. We rated that study as having a low risk of reporting bias 19. We rated the other trials as having an unclear risk of reporting bias because they did not report whether the study was registered or whether the study protocol was publicly assessable.
Fig. 2
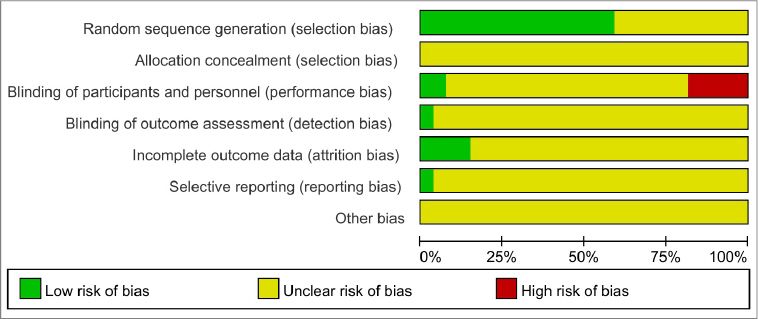
Fig. 3
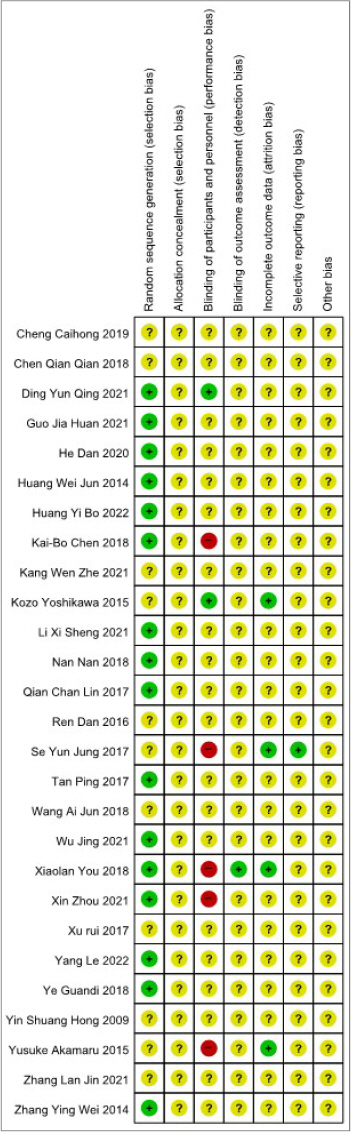
7) Other bias
Because of insufficient information to judge, we rated all of the studies as having an unclear risk of reporting bias.
3. Outcomes
1) Time to first flatus
Twenty-six studies reported data on time to the first flatus. There was a significant decrease in time to first flatus in the studies of Gami-leejoongtang 41, Sama-tang combined with warm needling 23,33, Gumiseunggi-tang combined with warm needling 24, Daeseunggi-tang combined with warm needling 38, Insam-tang combined with acupuncture 39, acupuncture 19,27,35, acupuncture and moxibustion 37, electroacupuncture 42, warm needling 30,40, TEA 17, TEAS 21, LEAS 22, ST-36 acupoint injection with neostigmine 16, moxibustion 28,31,34,36, and auricular acupressure 25,26,29,32. Meta-analysis results revealed significant decreases in the time to first flatus in the acupoint stimulation studies (MD=-15.62, 95% CI : -18.98 to -12.26, I 2=96%) ( Fig. 4).
Fig. 4
Forest plot of acupoint stimulation group versus control group: time to first flatus. 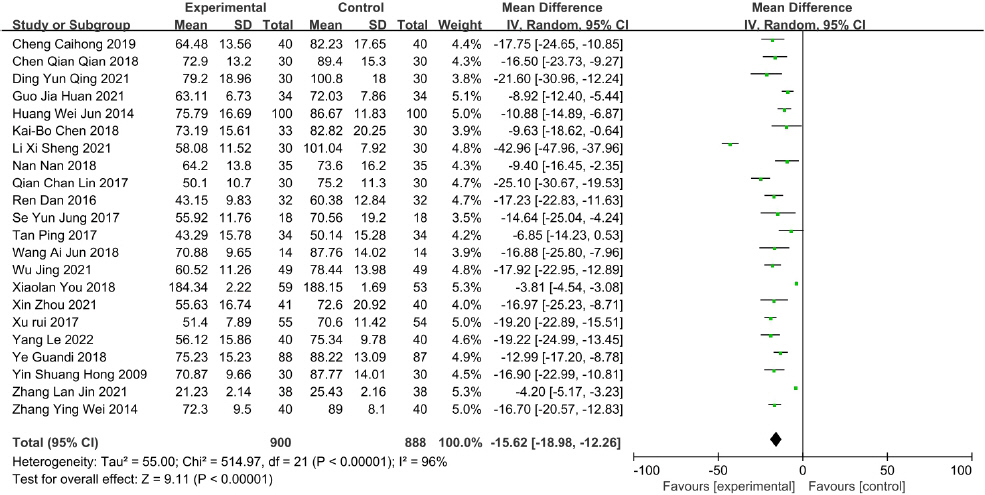
The TEA, TEAS, LEAS, and electroacupuncture study showed that the time to first flatus following surgery was significantly shorter in the TEA, TEAS, LEAS, and electroacupuncture groups than in the non-treatment groups 17,21,22,42 (p=0.038, p<0.001, p<0.05, p<0.01). The time to first flatus in the acupuncture trials was significantly shorter in the acupuncture groups than that in the non-acupuncture groups 19,27,35 (p=0.009, p<0.01, p<0.05). A significant decrease in the time to first flatus was reported in the treatment groups in the auricular acupressure studies 25,26,29,32, compared with the non-treatment groups (p<0.001, p<0.05, p=0.041, p<0.05). The time to first flatus in the Gami-leejoongtang trial was significantly shorter in the Gami-leejoongtang group than that in the non-treatment group 41 (p<0.05). In the warm needling studies 30,40, the time to first flatus after gastrectomy was shorter in the warm needling groups than in the non-treatment groups (p<0.01, p<0.01). The Sama-tang combined with warm needling studies reported that the time to first flatus was shorter in the Sama-tang combined with warm needling groups than in the non-treatment groups 23,33 (p<0.05, p<0.01). In the Gumiseunggi-tang combined with warm needling study, the time to first flatus following surgery was shorter in the Gumiseunggi-tang combined with warm needling group than in the Gumiseunggi-tang group 24 (p<0.05). The Daeseunggi-tang combined with warm needling study showed that the time to first flatus was shorter in the Daeseunggi-tang combined with warm needling group than in the warm needling group 38 (p<0.05). In the Insam-tang combined with acupuncture study 39, the time to first flatus was shorter in the treatment group than in the non-treatment group (p<0.05). The ST-36 acupoint injection study reported a significantly shorter time to first flatus in the ST-36 acupoint injection group than that in the standard therapy group, but it was not significantly shorter in the acupuncture group than in the standard therapy group (p=0.175). In this study, standard therapy was gradual ambulation with simple exercises, fasting, intravenous fluids, and parenteral nutrition 16. The moxibustion studies 28,31,34,36 showed that the time to first flatus was shorter in the moxibustion groups than in the non-treatment groups (p<0.01, p<0.05, p<0.01, p<0.05). In the acupuncture and moxibustion study 37, the time to first flatus was shorter in the acupuncture group and moxibustion group than in the non-treatment group (p<0.01, p<0.01). In the DKT study 18, the median time from the end of the operation (tracheal tube extubation) to the first flatus was slightly longer in the DKT group (68.9 h) than in the placebo group (68.3 h), but it was not significant.
2) Time to the first defecation
Twenty-one studies reported data on time to the first defecation. There was a significant decrease in time to the first defecation in the studies of Gami-leejoongtang 41, DKT 18, Sama-tang combined with warm needling 23,33, Gumiseunggi-tang combined with warm needling 24, Daeseunggi-tang combined with warm needling 38, Insam-tang combined with acupuncture 39, acupuncture 35, electroacupuncture 42, warm needling 30,40, TEAS 21, LEAS 22, ST-36 acupoint injection with neostigmine 16, moxibustion 31,36, and auricular acupressure 25,26,32. Meta-analysis results revealed significant decreases in the time to the first defecation in the acupoint stimulation studies (MD=-16.26, 95% CI : -19.66 to -12.86, I 2=97%) ( Fig. 5).
Fig. 5
Forest plot of acupoint stimulation group versus control group: time to first defecation. 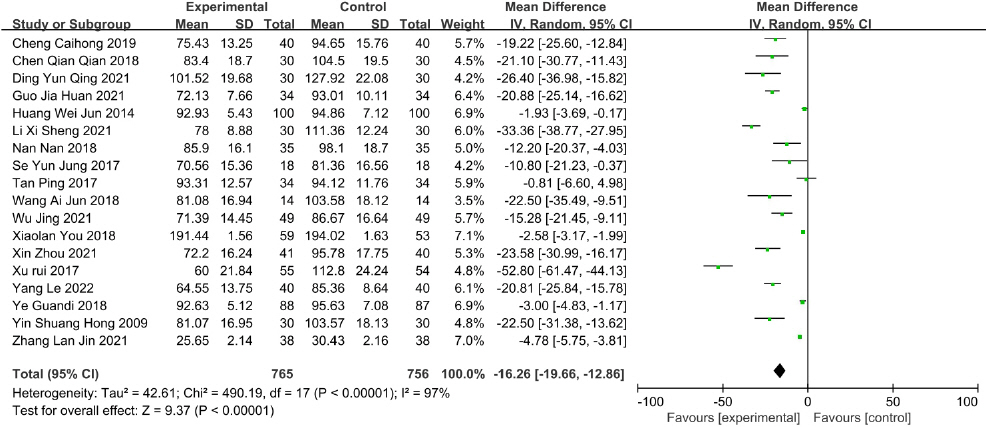
The TEAS, LEAS, and electroacupuncture trial showed that the time to the first defecation was shorter in the TEAS, LEAS, and electroacupuncture groups than in the non-treatment groups 21,22,42 (p<0.001, p<0.05, p<0.01). A significant decrease in the time to the first defecation was observed in the treatment groups in the Sama-tang combined with warm needling studies, compared with the non-treatment groups 23,33 (p<0.05, p<0.01). In the Gumiseunggi-tang combined with warm needling study, the time to the first defecation was significantly shorter in the Gumiseunggi-tang combined with warm needling group than in the Gumiseunggi-tang group 24 (p<0.05). The Daeseunggi-tang combined with warm needling study showed that the time to the first defecation was shorter in the Daeseunggi-tang combined with warm needling group than in the warm needling group 38 (p<0.05). A significant decrease in the time to the first defecation was reported in the treatment group in the Insam-tang combined with acupuncture trial, compared with the non-treatment group 39 (p<0.05). A significant decrease in the time to the first defecation was observed in the treatment group in the DKT study (94.7 h), compared with the placebo (113.9 h) (p=0.05) 18. In the Gami-leejoongtang study, the time to the first defecation was shorter in the Gami-leejoongtang group, compared with the non-treatment group 41 (p<0.05). The acupuncture trials reported that the acupuncture groups had a relatively faster time to first defecation (p=0.054, p<0.05) 19,35. In the auricular acupressure trials, the time to the first defecation was shorter in the treatment groups, compared with the non-treatment groups 25,26,29,32 (p=0.032, p<0.05, p=0.726, p<0.05). In the ST-36 acupoint injection with neostigmine study, the time to the first defecation was significantly shorter in the acupoint injection group than that in the standard therapy group (p<0.01), but it was not significantly shorter in the acupuncture group than the standard therapy group (p=0.256) 16. The warm needling trials reported that the time to the first defecation was shorter in the warm needling groups than in the non-treatment groups 30,40 (p=0.002, p<0.01). A decrease in the time to the first defecation was reported in the treatment groups in the moxibustion trials, compared with the non-treatment groups 31,36 (p<0.05, p<0.05).
3) Incidence of postoperative ileus
Three of 96 patients in the DKT group and two of 99 patients in the placebo group presented with POI in a study by Kozo Yoshikawa 18. One of 51 patients in the DKT group and two of 40 patients in the placebo group developed an intestinal obstruction within 3 months in a study by Yuseke Akamaru 20. There was no statistical difference in the incidence of POI between treatment groups and control groups from the above-mentioned RCTs using DKT. The TEA trial reported that the incidence of prolonged ileus was 0 in the TEA group and 3 in the non-TEA group (p=0.102), with prolonged POI defined in this study as not passing flatus for 5 days after the gastrectomy.
4) Adverse events
No serious adverse events were reported in any of the included RCTs.
IV. Discussion
Surgical treatment is recommended for gastric cancer patients, most of whom suffer from functional gastrointestinal disorders during the postoperative period. Although POI is common, inhibits early recovery, and increases the length of hospital stay, there is no exact etiology or treatment for POI. Several TKM treatment studies have reported that TKM improves bowel function after surgery. We conducted a systematic review and meta-analysis of the effectiveness of TKM treatment for POI in gastric cancer patients.
The time to first flatus in gastric cancer patients after gastrectomy was shortened by the TKM treatments, such as herbal medicine combined with acupuncture (Sama-tang, Gumiseunggi-tang, Daeseunggi -tang, Insam-tang), acupuncture, warm needling, moxibustion, auricular acupressure, TEAS, LEAS, TEA, electroacupuncture, and the acupoint injection (ST-36), which was demonstrated by the meta-analysis in this systematic review ( Fig. 4). The time to the first defecation decreased significantly in response to herbal medicine combined with acupuncture (Sama-tang, Gumiseunggi-tang, Daeseunggi-tang, Insam-tang), acupuncture, warm needling, TEAS, LEAS, electroacupuncture, moxibustion, auricular acupressure, and the acupoint injection (ST-36) ( Fig. 5). POI is believed to be mediated by many factors, including gastrointestinal motility, the autonomic nervous system, neurotransmitters, local factors, hormones, and inflammation, which control bowel motility 43. Thus, the complex interplay between these factors makes it difficult to understand the clear pathogenesis and determine an exact treatment for POI 44. The typically recommended approaches to alleviate POI include a nasogastric tube, early postoperative feeding, gum chewing, laparoscopic procedures, and pharmacological agents 43,44. However, until now, no single strategy has had a significant effect on POI 45. Thus, establishing a novel strategy to manage POI in patients with gastric cancer is important. Acupuncture can directly restore gastrointestinal transit via the parasympathetic efferent pathway 13,46. In addition, acupuncture increases postoperative bowel motility by activating the interstitial cells of Cajal (ICCs) 47,48. ICCs, the pacemaker cells of gastrointestinal motility, generate and propagate slow electrical waves and act as mediators that transmit enteric neural input to smooth muscle cells 49. Recent studies 47,50 have demonstrated that electroacupuncture (EA) decreases intestinal inflammation, which is the main factor associated with delayed dysfunction of the gastrointestinal tract 51. EA activates the 7nAChR-mediated Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway in POI. DKT is a traditional herbal medicine composed of dried pepper, processed ginger, ginseng radix, and maltose powder 18. DKT has been used to treat gastrointestinal disorders, including adhesive ileus and paralytic ileus 52-58. DKT has been recently reported to significantly inhibit cyclooxygenase-2 activity 59 and downregulate various inflammatory mediators, including tumor necrosis factor-α, interleukin-1β, and endothelin-1. Mechanistic studies on the TKM treatments mentioned above are thought to lay the theoretical foundation for the RCT results included in this systematic review. This study had some limitations. The first limitation is that except two studies 18,42, the majority of the trials were not blinded to the physicians and participants or there was no statement about blinding, which is related to preventing bias because of the demanding characteristics or placebo effects. Furthermore, the databases considered in this study were limited to English, Japanese, Chinese, and Korean literature, which may have contributed to bias. In conclusion, this study suggests that acupuncture, TEA, TEAS, LEAS, electroacupuncture, DKT, Gami-leejoongtang, Sama-tang combined with warm needling, Gumiseunggi-tang combined with warm needling, Daeseunggi-tang combined with warm needling, Insam-tang combined with acupuncture, warm needling, moxibustion, auricular acupressure, and acupoint injection with neostigmine improved the gastrointestinal function of gastric cancer patients after gastrectomy during the postoperative recovery period compared with control interventions. Thus, TKM should be considered a promising option for preventing and resolving POI in gastric cancer patients. Further well-designed studies are needed to clarify these findings and determine better interventions for POI.
Acknowledgement
This research was supported by the Daejeon University fund (2021).
References
1. Sung HA, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal , et al. Global Cancer Statistics 2020:globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021:71(3):209–49.    2. Korenaga D, Okamura T, Baba H, Saito A, Sugimachi K. Results of Resection of Gastric Cancer Extending to Adjacent Organs. Br J Surg 1988:75(1):12–5.    3. Martin R, Jaques D, Brennan M, Karpeh M. Achieving RO Resection for Locally Advanced Gastric Cancer:is it worth the risk of multiorgan resection? J Am Coll Surg 2002:194(5):568–77.  4. Liedman B. Symptoms after Total Gastrectomy on Food Intake, Body Composition, Bone Metabolism, and Quality of Life in Gastric Cancer Patients—Is Reconstruction with a Reservoir Worthwhile? Nutrition 1999:15(9):677–82.   5. Waldhausen JHT, Shaffrey ME, Skenderis BS, Jones RS, Schirmer BD. Gastrointestinal Myoelectric and Clinical Patterns of Recovery after Laparotomy. Ann Surg 1990:211(6):777–85.    6. Artinyan A, Nunoo-Mensah JW, Balasubramaniam S, Gauderman , J , Essani , R , Gonzalez-Ruiz C, et al. Prolonged Postoperative Ileus - Definition, Risk factors, and Predictors after Surgery. World J Surg 2008:32(7):1495–500.    7. Vather R, Trivedi S, Bissett I. Defining Postoperative Ileus:Results of a Systematic Review and Global Survey. J Gastrointest Surg 2013:17(5):962–72.    8. Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, et al. Consensus Guidelines for Enhanced Recovery after Gastrectomy:enhanced recovery after surgery (ERAS®) society recommendations. Br J Surg 2014:101(10):1209–29.  9. Iyer S, Saunders WB, Stemkowski S. Economic Burden of Postoperative Ileus Associated. J Manag Care Pharm 2009:15(6):485–94.  10. Kehlet H. Fast-track colorectal surgery. Lancet 2008:371(9615):791–3.   11. Miedema BW, Johnson JO. Methods for Decreasing Postoperative Gut Dysmotility. Lancet Oncol 2003:4(6):365–72.   12. Wattchow D, Heitmann P, Smolilo D, Spencer NJ, Parker D, Hibberd T, et al. Postoperative Ileus—An Ongoing Conundrum. Neurogastroenterol Motil 2021:33(5):1–11.    14. Ishizuka M, Shibuya N, Nagata H, Takagi K, Iwasaki Y, Hachiya H, et al. Perioperative Administration of Traditional Japanese Herbal Medicine Daikenchuto Relieves Postoperative Ileus in Patients Undergoing Surgery for Gastrointestinal Cancer:a systematic review and meta-analysis. Anticancer Res 2017:37(11):5967–74.  16. You X, Wang Y, Wu J, Liu Q, Liu Y, Qian Y, et al. Zusanli (ST36) Acupoint Injection with Neostigmine for Paralytic Postoperative Ileus Following Radical Gastrectomy for Gastric Cancer:a randomized clinical trial. J Cancer 2018:9(13):2266–74.    17. Chen KB, Lu YQ, Chen JD, Shi DK, Huang ZH, Zheng YX, et al. Transcutaneous Electroacupuncture Alleviates Postoperative Ileus after Gastrectomy:a randomized clinical trial. World J Gastrointest Surg 2018:10(2):13.    18. Phase P, Yoshikawa K, Shimada M, Wakabayashi G, Ishida K, Kaiho T, et al. Effect of Daikenchuto, a Traditional Japanese Herbal Medicine, after Total Gastrectomy for Gastric Cancer:a multicenter, randomized, double-blind, placebo-controlled, phase II trial. Journal of the American College of Surgeons 2015:221(2):571–8.   20. Akamaru Y, Takahashi T, Nishida T, Omori T, Nishikawa K, Mikata S, et al. Effects of Daikenchuto, a Japanese Herb, on Intestinal Motility After Total Gastrectomy:a prospective randomized trial. J Gastrointest Surg 2015:19(3):467–72.    22. He Dan, Wang FZ, Zhang Z, Huang F, Chen JJ, Li B. Effect of Low-frequency Electrical Acupoint Stimulation on Gastrointestinal Motility Function Following Radical Gastrectomy in Patients with Gastric Cancer. Acupunct Res Jan 2020:45(1):51–6.
23. Chen QQ. Effect of Simotang Oral Liquid and Warm Acupuncture on Gastrointestinal Function and Peripheral Blood Leukocyte after Operation in Patients with Gastric Cancer. J Qiqihar Med Univ 2015:3(April):49–58.
24. Cheng CH. Effect of Jiuwei Chengqi Oral Liquid Combined with Warm Acupuncture and Moxibustion on Gastrointestinal Function and Peripheral Leukocytes in Middle-aged and Elderly Patients with Gastric Cancer after Total Gastrectomy. CJGMCM Novemb 2019:34(21):3279–81.
25. Huang WJ, Duan PB, Wang XQ, Zhu JH, Wu YH, Sun L, et al. The Effectiveness of Auricular Pressure on Gastrointestinal Function Recovery of Postoperative Patients with Gastric Cancer. J Nurs Adm 2014:14(11):827–9.
26. Nan N, Zhang Y, Lu HS. Effect of Auricular Acupressure on Gastrointestinal Function and Serum Gastrointestinal Hormone after Radical Gastrectomy. China Mod Dr 2018:56(32):92–4. 98.
27. Qian CL, Liu H, Zhang J, Qiu WQ, Shen ZY, Sun JH. Clinical Study on Acupuncture at Lower He-Sea Point for Promoting Functional Recovery after Stomach Cancer Surgery. Shanghai J Acupunct Moxibustion 2017:36(9):1044–48.
28. Ren D. Clinical Effect of Moxibustion on Abdominal Distension after Gastric Cancer Operation. J new Chinese Med Novemb 2016:48(11):184–5.
29. Tan P, You JH, Chen Q, Cai FY, Zhou JX, Huang XF. Influence of Ear Acupoint Pressing Beans on Gastrointestinal Function in Elderly Patients after Gastric Cancer Operation. Chinese Nurs Res December 2017:31(35):4562–4.
30. Wang AJ. Effect of Warm Needling on Gastrointestinal Function Recovery after Gastrointestinal Cancer Operation. Med Equipment 2018:31(4):125–6.
31. Xu R, Xiu MN, Li M, Fu HX, Zou JR. Clinical Study on Moxibustion and Ligitongfufang Footbath for Promoting Gastrointestinal Functional Recovery of Postoperative Gastric Cancer Patients. Jiangsu J Tradit Chinese Med 2017:49(12):62–4.
32. Ye GD, Pan A, Xu HT. The Value of Auricular Acupressure in Promoting the Rapid Recovery of Gastrointestinal Function in Patients with Gastric Cancer after Surgery. China Mod Dr Novemb 2018:56(33):153–6.
33. Yin SH, Du YQ, Liu B. Clinical Study on Acupuncture Combined with Medication in Restoration of Gastrointestinal Functions for Postoperative Patients with Gastric Cancer. Chinese Acupunct Moxibustion 2009:29(6):459–62.
34. Zhang YW, Li AY, Ye JY. Effect of Moxibustion on Recovery of Gastrointestinal Motility Function after Gastric Cancer Operation. Nurs Rehabil J 2014:8:795–6.
35. Yang L, Wu X, Huang J. Effect of Acupuncture for Regulating Qi and Unblocking Fu-Organs on Postoperative Gastrointestinal Function Recovery of Patients After Radical Gastrectomy for Gastric Cancer. New Chinese 2022:54(9):143–7.
36. Zhang LJ, Wu JN. Effect of Timing Moxibustion on Recovery of Gastrointestinal Function in Patients with Gastric Cancer after Operation. Chinese and Foreign Medical Research 2021:19(30):166–8.
37. Kang WZ, Li Y, Ma FH, Tian Z, Zuo WH, Ma FH, et al. Evaluation of acupoint acupuncture and moxibustion for promotion of recovery of gastrointestinal function after laparoscopic radical gastrectomy for gastric cancer. Chin J Clin Oncol Rehabil 2021:28(10):1158–61.
38. Li XS, Bai L. Efficacy of Dachengqi decoction combined with acupuncture for recovery of gastrointestinal function and function of the immune system after surgery for gastric cancer. Chin J Clin Oncol Rehabil 2021:28(11):1367–70.
39. Guo JH. The Effect of Renshen Decoction Combined with Acupuncture on the Recovery of Gastrointestinal Function in Patients with Gastric Cancer after Operation. Chinese Med Mod Distance Educ China 2021:19(16):120–2.
40. Wu J, Zhang H, Mao F. Effects of Warm Acupuncture Combined with Acupoint Massage on Serum Gastrin Motilin Level and Gastrointestinal Function in Patients with Gastric Cancer after Operation. Chin J Clin Res 2021:34(2):232–5.
41. Huang YB, Niu YQ. Curative Effect of Modified Lizhong Tang for Gastrointestinal Dysfunction after Operation for Gastric Cancer and its Effect on Early Recovery of Gastrointestinal Function. J New Chinese Med 2022:54(1):150–3.
42. Ding YQ, Huang T. Electroacupuncture treatment in gastric cancer patients:a RCT research for enhanced recovery after surgery. Journal of Baotou Medical College 2021:37(3):107–10.
43. Luckey A, Livingston E, Tache Y. Mechanisms and Treatment of Postoperative Ileus. Arch Surg 2003:138(2):206–14.   44. Khawaja ZH, Gendia A, Adnan N, Ahmed J. Prevention and Management of Postoperative Ileus:a review of current practice. Cureus 2022:14(2):e22652.    45. Bowker B, Calabrese RO, Barber E. Postoperative Ileus. Physician Assist Clin 2021:6(2):215–27.  46. Okada M, Itoh K, Kitakoji H, Imai K. Mechanism of Electroacupuncture on Postoperative Ileus Induced by Surgical Stress in Rats. Med Acupunct 2019:31(2):109–15.    47. Deng J, Yang S, Yuan Q, Chen Y, Li D, Sun H, et al. Acupuncture Ameliorates Postoperative Ileus via IL-6-miR-19a-KIT Axis to Protect Interstitial Cells of Cajal. Am J Chin Med 2017:45(4):737–55.   48. Deng JJ, Lai MY, Tan X, Yuan Q. Acupuncture Protects the Interstitial Cells of Cajal by Regulating miR-222 in a Rat Model of Post-operative Ileus. Acupunct Med 2019:37(2):125–32.    49. Hulzinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene Required for Interstitial Cells of Cajal and for Intestinal Pacemaker Activity. Nature 1995:373(6512):347–49.    50. Yang NN, Ye Y, Tian ZX, Ma SM, Zheng Y, Huang J, et al. Effects of Electroacupuncture on the Intestinal Motility and Local Inflammation are Modulated by Acupoint Selection and Stimulation Frequency in Postoperative Ileus Mice. Neurogastroenterol Motil 2020:32(5):e13808.    51. Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, et al. Crosstalk between Muscularis Macrophages and Enteric Neurons Regulates Gastrointestinal Motility. Cell 2014:158(2):300–13.    52. Han GJ, Jang MW, Seong S, Kim SS. A Case Report of Chemotherapy-Induced Hand-Foot Syndrome Treated with Modified Dohongsamul-tang. J Intern Korean Med 2018:39(2):259–67.   53. Sakakibara R, Odaka T, Lui Z, Uchiyama T, Yamaguchi K, Yamaguchi T, et al. Dietary Herb Extract Dai-kenchu-to Ameliorates Constipation in Parkinsonian Patients (Parkinson's disease and multiple system atrophy). Mov Disord 2005:20(2):261–2.    54. Ohya T, Usui Y, Arii S, Iwai T, Tsunoda S. Effect of Dai-kenchu-to on Obstructive Bowel Disease in Children. Am J Chin Med 2003:31(1):129–35.   55. Iwai N, Kume Y, Kimura O, Ono S, Aoi S, Tsuda T. Effects of Herbal Medicine Dai-Kenchu-To on Anorectal Function in Children with Severe Constipation. Eur J Pediatr Surg 2007:17(2):115–8.   56. Nakamura T, Sakai A, Isogami I, Noda K, Ueno K, Yano S. Abatement of Morphine-Induced Slowing in Gastrointestinal Transit by Dai-kenchu-to, a Traditional Japanese Herbal Medicine. Jpn J Pharmacol 2002:88(2):217–21.   57. Furukawa Y. Effects of the Chinese Herb Medicine “Dai-kenchu-to”on GI Motility and for Treatment of Bowel Obstruction. Gastroenterology 1999:116:A811.
58. Satoh K, Kase Y, Yuzurihara M, Mizoguchi K, Kurauchi K, Ishige A. Effect of Dai-kenchu-to (Da-Jian-Zhong-Tang) on the Delayed Intestinal Propulsion Induced by Chlorpromazine in Mice. J Ethnopharmacol 2003:86(1):37–44.   59. Hayakawa T, Kase Y, Saito K, Hashimoto K, Ishige A, Komatsu Y, et al. Effects of Dai-kenchu-to on Intestinal Obstruction Following Laparotomy. J Smooth Muscle Res 1999:35(2):47–54.  
|
|
















