I. Introduction
Recently, the incidence of obesity accompanying type II diabetes (T2DM), which is attributed by physical inactivity and high-caloric diet intake1, 422 million global people were suffering from diabetes in 2014 and a constant increase in prevalence of diabetes is expected2. Increase of regional body fat distribution, especially abdominal fat, would be one of the critical sources of the obesity development. Abdominal obesity often accompanies atherogenic risk factors, such as hypertension, dyslipidemia, insulin resistance, and changes in inflammatory and coagulation cytokine profiles3,4. As the outcomes, cardiovascular disease morbidity and mortality are increased5. As lipid accumulation proceeds, adipokines, which affect inflammation and lipid metabolism, are secreted by adipose tissue. Therefore, modulation of adipose endothelial cell can alleviate chronic state of inflammation, which may lead to complications including insulin resistance6. However, multi-drugs applied for diabetes and related complications not only manifest inconveniency to patients, but also variety of adverse effects and limitations.
As increasing prevalence of diabetes and related complications, various protective or therapeutic methods have been developed for the treatment. Various single herbal medicines have been verified of their effects on diabetes and related complications. Former studies have shown not only amelioration of diabetes, dyslipidemia and obesity, but also regulation of mRNA expression about lipid metabolism. However, studies about herbal medicines are insufficient. Since there are a number of limitations in currently available pharmacological therapies for diabetes and related complications due to high rates of secondary failure and adverse effects7, alternative and complementary approaches are required8. Korean traditional medicines are characterized by global regulation of glucose metabolism and energy homeostasis via multiple ingredients in a prescription. Therefore, it is valuable to explore novel drugs with less negative effects from herbal extracts, compared to synthetic drugs targeting hyperglycemia, insulin resistance and diabetes. Geumnyeonyijin-tang (GNYJT) is a well-documented traditional herbal formula, listed in “Uihag-ibmun”, which consists of Yijin-tang (YJT), Scutellariae Radix and Coptidis Rhizoma. Until now, satisfactory effects of YJT9, Scutellariae Radix10 and Coptidis Rhizoma10 on diabetes and related complications have been revealed. However, there are no systemic in vivo experiments dealing efficacies of GNYJT, itself, on the diet induced diabetes and related complications.
Therefore, referring to indice used for evaluation of treatment efficacy in previous studies, I conducted an experimental study about GNYJT, focusing on its multi-target preventive effects and expressing possibility of therapeutic agent for diabetes and related complications. We intended to confirm the dose-dependent beneficial potentials of 400, 200 and 100 mg/kg of GNYJT water extracts on mild diabetic obese mice, compared with those of 250 mg/kg metformin, which is a typical antiobesic and antidiabetic drug for T2DM.
II. Materials and Methods
1. Animals and husbandry
85 female specific pathogen free (SPF)/ virus antibody free (VAF) CrljOri:CD1[ICR] mice in total (6-week-old on arrival to laboratory; OrientBio, Seungnam, Korea), were used in exam after 10 days of adaptation. Animals were supplied 45%/Kcal HFD (Cat. No. D12451; Research Diet, New Brunswick, NJ, USA) free to access after 10 days of adaptation. Instead of high fat diet (HFD), normal pellet diet (NFD) (Cat. No. 38057; Purinafeed, Seungnam, Korea) was supplied free to access in intact control mice. Resultingly, total 48 mice were divided in 6 groups, which is 1 NFD group and 5 HFD groups. All animals in laboratory were treated according to the national managements of the usage and welfare of laboratory animals, and were approved by the Institutional Animal Care and Use Committee in Daegu Haany University (Gyeongsan, Gyeongbuk, Korea) prior to animal experiment [Approval No. DHU2020-018, April 13, 2020].
2. Preparations and administration GNYJT water extracts
GNYJT water extracts (yield=17.38%), appearing as deep brown powders, were arranged by routine methods utilizing programmable freeze dryer (FDB-5503, Operon, Kimpo, Korea) and rotary vacuum evaporator (N-1110, Eyela, Tokyo, Japan). They were arranged from 5-fold (Total 295 g) of traditional composition of GNYJT, 7 types of natural herbs (Total 59 g) - Pinelliae Tuber (15 g), Citri Unshius Pericarpium (15 g), Poria Sclerotium (9 g), Glycyrrhizae Radix Rhizoma (5 g), Zingiberis Rhizoma Recens (3 g), Scutellariae Radix (6 g) and Coptidis Rhizoma (6 g), which were purchased from local seller (Jecheon Hanbang Yakcho, Jecheon, Korea) after the confirmation of morphology under microscopy. White powders of metformin hydrochloride (Wako, Osaka, Japan) were applied as reference drugs. In this study, 400 mg/kg was selected as the highest dosages of GNYJT water extracts based on the traditional clinical dosages, and the middle and lowest dosages of GNYJT water extracts were decided as 200 and 100 mg/kg using common ratio 2, respectively. The administration volume was decided as 10 mL/kg, a general dosing volume in mice11, respectively. The dosage levels of 250 mg/kg metformin were also chosen based on previous animal studies8,12-16. Appropriate amounts of GNYJT water extracts were directly dissolved in distilled water at concentrations of 40, 20 and 10 mg/mL, and were orally treated in a 10 mL/kg volume, equivalent to 400, 200 and 100 mg/kg, daily for 84 days, using a stainless zonde which was attached to 1 mL syringe, after first week of HFD supply. In addition, metformin was dissolved in distilled water as concentration of 25 mg/mL and was also orally administered in 10 mL/kg volume, equivalent to 250 mg/kg, daily for 84 days after 1 week of initial HFD supply. In intact and HFD vehicle control mice, oral administrations of distilled water in equal volumes were conducted, instead of test substances in order to provide same restraining stresses to gastric gavages, respectively.
3. Measurement of body weight changes, mean daily food consumption and body fat mass: total and abdominal fat mass (%)
Body weight changes were checked at 8 days and 1 day before beginning of administration, once a week starting from the first day of administration. Additionally, body weight gains were calculated during adaptation periods and medicating periods.
Diets were provided, and remainder amounts of provided diets were measured after 24 hrs. On next stage, the measured amounts were divided by raised animal numbers in each cage. These results are considered as average daily food consumption of individual mice (g/day/mice).
The mean fat mass of the abdominal and total body cavities of each mouse were detected only at termination of test substances administration.
4. Measurement of blood glucose level
At the termination of administration, blood samples were taken from vena cava. Plasma were separated, and stored in a ultradeep freezer (MDF-1156, Sanyo, Tokyo, Japan) under -150 °C until analysis. Blood glucose levels were assessed utilizing automated blood analyzer (Dri-Chem NX500i; Fuji Medical System Co., Ltd., Tokyo, Japan).
5. Serum biochemistry, and measurement of serum insulin and blood glycated hemoglobin, hemoglobin A1c (HbA1c) level
Some blood samples collected from vena cava after 84 days from initial administration under anesthesia, were placed in clotting activated serum tubes, and were centrifuged at 15,000 rpm for 10 min under room temperature to separate the serum to total cholesterol (TC), triglyceride (TG), low density lipoprotein (LDL) and high density lipoprotein (HDL) measurement. Serum insulin and HbA1c levels were measured using an automated HbA1c measuring equipment (Model Easya1c; Infopia, Anyang, Korea), and a mouse insulin enzyme linked immunosorbent assay (ELISA) kit (Cat. No. 80-INSMS-E01; Alpco Diagnostics, Windham, NH, USA).
6. Organ weight measurements and lipid compositions in the feces
At the time of sacrifice, the weights of liver, abdominal wall fat pads attached to the muscularis quadratus lumborum and left periovarian fat pads were measured by gram levels, separately. Also, in order to minimize the differences between individual body weights, the absolute weights and relative weights (% of body weights) were also calculated applying body weight at the time of sacrifice.
Lipid was extracted from feces, which were collected 8 hrs. after last administration. The concentrations of fecal TG and TC were measured by colorimetric assay.
7. Measurement of hepatic glucose-regulating enzyme activities
Hepatic tissue 0.3 g was homogenized in buffer solution (0.002 M dithiothreitol, 0.2 M EDTA and 0.1 M triethanolamine) and was centrifuged for 15min, at 1,000×g, 4 °C. The glucokinase (GK) activity was assessed based on the method described by Davidson and Arion17 with minimal modifications. The activity of G6pase was assessed according to the method of Alegre et al18. The activity of phosphoenolpyruvate carboxykinase (PEPCK) was assessed based on the method of Bentle and Lardy19. All chemicals and reagents used in this measurement of hepatic enzyme activity were acquired from Sigma-Aldrich (St. Louise, MO, USA).
8. Realtime reverse transcriptase polymerase chain reaction (RT-PCR) analysis
The 5’ adenosine monophosphate-activated protein kinase (AMPK)α1, AMPKα2 and acetyl-CoA carboxylase 1 (ACC1) mRNA expressions on the arranged hepatic tissues were assessed by realtime RT-PCR, with periovarian adipose tissue mitochondrial uncoupling protein (UCP)2, adiponectin, leptin, peroxisome proliferator-activated receptor (PPAR)α, PPARγ, fatty acid synthase (FAS), sterol regulatory element-binding protein (SREBP)1c, CCAAT-enhancer -binding protein (C/EBP)α and C/EBPβ mRNA expressions, individually based on the previously reported studies8,12,20.
9. Histopathology
After measurement of organ weights, left lateral lobes of liver, left periovarian fat pads, splenic lobes of abdominal wall fat pads which are attached to the quadratus lumborum muscle were fixed with 10% neutral buffered formalin. Representative sections were marked with hematoxylin and eosin (HE) for light microscopic examination. Alternatively, portions of liver that had been dehydrated with 30% sucrose solutions were segmented by cryostat for oil red staining8,12,16,21,22. To observe more detailed histopathological changes, mean hepatocyte diameters (under HE staining) were measured by automated image analysis process on the restricted view fields according to previous method8,12,13,15,16,21,22.
10. Statistical analyses
All numerical values were expressed in average ±standard deviation (SD) of 8 mice. Various comparison tests for each group were carried out. Variance of homogeneity was evaluated using the Levene test23. If the Levene test revealed no significant difference from variance of homogeneity, the acquired data were assayed by one way analysis of variance (ANOVA) test followed by Tukey’s Honest Significant Difference (THSD) test to evaluate the significant difference in pairs of group comparison. In case that significant difference from variance of homogeneity were noted at Levene test23, Kruskal-Wallis H test, a non-parametric comparison test, was conducted. When a significant deviation has been in the Kruskal-Wallis H test, the Mann-Whitney U (MW) test was processed to discover the significant difference of the specific pairs of group comparison. Statistical analyses were processed by SPSS for Windows (Release 14.0K, IBM-SPSS Inc., Chicago, IL, USA)24. Additionally, the change of percentage in comparison to HFD control in GNYJT water extracts 100, 200 and 400 mg/kg or metformin 250 mg/kg orally treated mice were measured in order to help the awareness of the efficacy of test substances, and the percentage changes between HFD control and NFD supplied intact were also measured to determine disease inductions, regarding previous report8,12.
III. Results
1. Effects on obesity
1) Effects on the body weight changes and food consumption
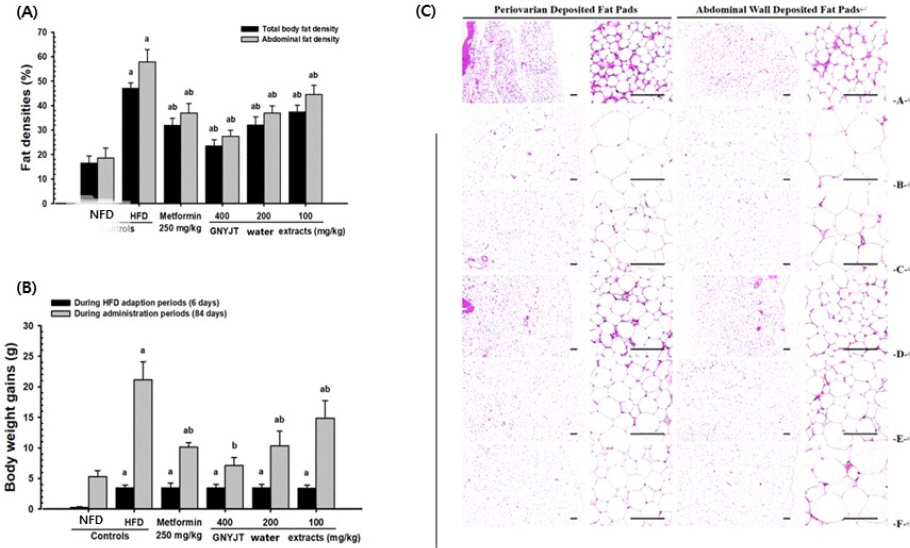
The body weight gains throughout 84 days of administration were also reduced dose-dependently and significantly (p<0.01) in every three different dosages of GNYJT water extract and metformin 250 mg/kg treated mice, in comparison to those of HFD control mice, respectively. Especially, GNYJT water extracts 200 mg/kg showed similar inhibitory activities on HFD-induced body weight and gain increases in comparison to those of 250 mg/kg metformin (Fig. 1-A).
Fig. 1
Body weight gains & total body and abdominal fat densities & representative histological images of the adipocytes in NFD or HFD supplied mice.
Values are expressed as Mean±SD of 8 mice.
a p<0.01 as compared to intact control by THSD test b p<0.01 as compared to HFD control by THSD test
(A) Body weight gains in NFD or HFD supplied mice.
(B) Total body and abdominal fat densities in NFD or HFD supplied mice.
(C) Representative histological images of the adipocytes, taken from NFD or HFD supplied mice periovarian and abdominal wall deposited fat pads.
A=10 mL/kg of vehicle (distilled water) orally applied mice with supply of NFD (NFD control)
B=10 mL/kg of vehicle orally applied mice with supply of HFD (HFD control)
C=250 mg/kg of metformin orally treated mice with supply of HFD (Metformin)
D=400 mg/kg of GNYJT water extracts orally treated mice with supply of HFD (GNYJT400)
E=200 mg/kg of GNYJT water extracts orally treated mice with supply of HFD (GNYJT200)
F=100 mg/kg of GNYJT water extracts orally treated mice with supply of HFD (GNYJT100)
Scale bars=80 µm.
NFD : normal pellet diet, HFD : high fat diet, GNYJT : Geumnyeonyijin-tang, THSD : Tukey’s honest significant difference
Although significant (p<0.01) decreases of mean daily food consumptions were detected in HFD control mice in comparison to intact control, no meaningful or significant changes on the mean daily food consumptions were detected in all test substance administered groups including metformin 250 mg/kg in comparison to HFD control.
2) Effects on the body fat mass: total and abdominal fat mass (%)
Significant (p<0.01) expansion of abdominal fat and total body mass were observed in HFD control in comparison to intact control, respectively. On the contrary, significant (p<0.01) and dose-dependent reduction of abdominal and total body fat masses were noticed in all three different dosages of GNYJT water extracts in comparison to those of HFD control mice, also by 250 mg/kg metformin, respectively. Especially, GNYJT water extracts 200 mg/kg showed similar inhibitory activities against HFD-induced increases of total body and abdominal fat densities in comparison to 250 mg/kg of metformin (Fig 1-B).
3) Effects on the abdominal wall and periovarian fat pad weights
Significant (p<0.01) increases in weight of abdominal wall and periovarian fat pad were observed in HFD control group in comparison to intact control group (Table 1, Fig. 1-B).
Table 1
Changes on Absolute and Relative Organ Weights in NFD or HFD Supplied Mice
Significant (p<0.01) increases in abdominal and periovarian white adipocyte thicknesses and diameters of each fat pad were observed in HFD control in comparison to intact control, individually. But the hypertrophy of fat and adipocytes depositions were significantly (p<0.01) impeded by administration of all three different amounts of GNYJT water extracts, dose-dependently, in comparison to those of HFD control mice, also by 250 mg/kg metformin, respectively (Fig. 1-C).
2. Antidiabetic effects
1) Effects on the serum glucose, insulin, HbA1c levels and HOMA-IR
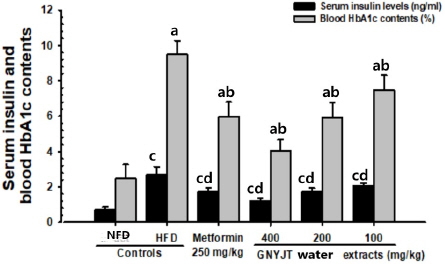
The serum glucose, insulin and HbA1c levels were significantly (p<0.01) lowered by administration of all three different dosages of GNYJT water extracts and metformin 250 mg/kg in comparison to those of HFD control mice, dose-dependently. Especially, GNYJT water extracts 200 mg/kg showed similar inhibitory activities against HFD-induced hyperglycemia in comparison to metformin 250 mg/kg (Table 2, Fig. 2). In addition, insulin resistance was calculated using homeostasis assessment model -insulin resistance (HOMA-IR) according to the formula: (Fasting insulin, uIU/mL)*(Fasting glucose, mg/dL) / 40525. It has shown the dose-dependent decrease of HOMA-IR in GNYJT groups, with similar insulin resistance with metformin and GNYJT 200 m/kg medication.
Table 2
Changes on Blood Glucose Levels in NFD or HFD Supplied Mice
Fig. 2
Serum insulin and blood HbA1c contents in NFD or HFD supplied mice.
Values are expressed mean±SD of 8 mice.
a p<0.01 as compared to intact control by THSD test
b p<0.01 as compared to HFD control by THSD test
c p<0.01 as compared to intact control by MW test
d p<0.01 as compared to HFD control by MW test
NFD : normal pellet diet, HFD : high fat diet, GNYJT : Geumnyeonyijin-tang, THSD : Tukey’s honest significant difference, MW : Mann-Whitney U
3. Effects on dyslipidemia
1) Effects on the serum TC, TG, LDL and HDL levels
Significant reduction (p<0.01) of the serum TC, TG and LDL levels, significant elevation (p<0.01) of the serum HDL level were detected all three different dosages of GNYJT water extracts and metformin 250 mg/kg in comparison to those of HFD control group, dose-dependently. Especially, GNYJT water extracts 200 mg/kg showed similar inhibitory activities against HFD-induced serum TC, TG, LDL level increases and serum HDL decrease in comparison to metformin 250 mg/kg (Table 3).
Table 3
Changes on Serum Lipid Contents in NFD or HFD Supplied Mice
2) Effects on the fecal TC and TG contents
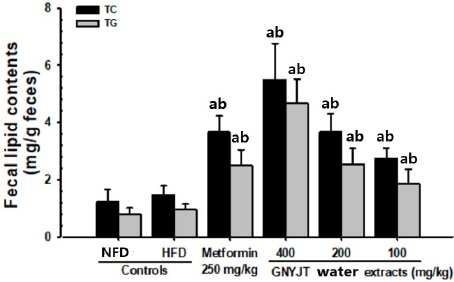
The TC and TG contents in all three different dosages of GNYJT water extracts and metformin 250 mg/kg were significantly (p<0.01) increased in comparison to those of HFD control mice, dose- dependently. Especially, GNYJT water extracts 200 mg/kg showed noticeable effects on fecal TG and TC contents, their excretions, in comparison to metformin 250 mg/kg (Fig. 3).
Fig. 3
Fecal total cholesterol and TG content in NFD or HFD supplied mice.
Values are expressed mean±SD of 8 mice.
a p<0.01 as compared to intact control by MW test
b p<0.01 as compared to HFD control by MW test
NFD : normal pellet diet, HFD : high fat diet, GNYJT : Geumnyeonyijin-tang, THSD : Tukey’s honest significant difference, MW : Mann-Whitney U
4. Effects on hepatopathy
1) Effects on the liver weights
The elevations of absolute liver weights were significantly (p<0.01) regulated by administration of all three different amounts of GNYJT water extracts and metformin 250 mg/kg in comparison to those of HFD control mice, dose-dependently (Table 1).
2) Effects on the steatohepatitis
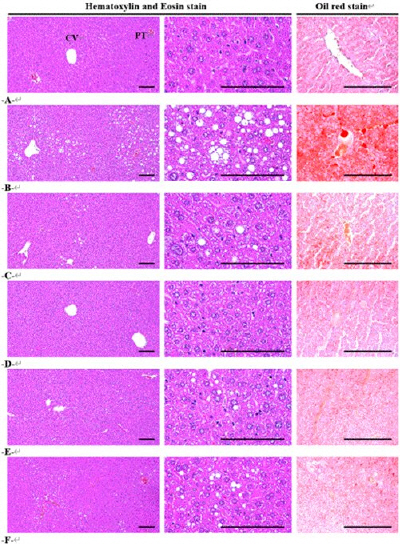
The oil red stain based histopathological steatohepatitis was significantly (p<0.01) stabilized by administration of all three different dosages of GNYJT water extracts and metformin 250 mg/kg in comparison to those of HFD control mice, dose-dependently. Especially, GNYJT water extracts 200 mg/kg showed similar inhibitory activities against HFD-induced steatohepatitis at histopathological levels in comparison to metformin 250 mg/kg (Table 4, Fig. 4).
Table 4
Changes on Histopathology-Histomorphometry of the Liver in NFD or HFD Supplied Mice
Fig. 4
Representative histological images of the liver, taken from NFD or HFD supplied mice.
A=10 mL/kg of vehicle (distilled water) orally applied mice with supply of NFD (NFD control)
B=10 mL/kg of vehicle orally applied mice with supply of HFD (HFD control)
C=250 mg/kg of metformin orally treated mice with supply of HFD (Metformin)
D=400 mg/kg of GNYJT water extracts orally treated mice with supply of HFD (GNYJT400)
E=200 mg/kg of GNYJT water extracts orally treated mice with supply of HFD (GNYJT200)
F=100 mg/kg of GNYJT water extracts orally treated mice with supply of HFD (GNYJT100)
Scale bars=80 μm.
NFD : normal pellet diet, HFD : high fat diet, GNYJT : Geumnyeonyijin-tang
3) Effects on the hepatocyte hypertrophy
The hepatocyte hypertrophies were noticeably and significantly (p<0.01) reduced in all three different dosages of GNYJT water extracts and metformin 250 mg/kg in comparison to those of HFD control mice, dose-dependently. Especially, GNYJT water extracts 200 mg/kg showed similar inhibitory activities against HFD-induced hepatocyte hypertrophies in comparison to metformin 250 mg/kg, at current histopathological level (Table 4, Fig. 4).
5. Effects on hepatic glucose-regulating enzyme activities
1) Effects on the hepatic GK, G6pase and PEPCK activity
The hepatic GK activities were significantly (p<0.01) increased, hepatic G6pase and PEPCK activities were significantly (p<0.01) decreased by treatment of all three different dosages of GNYJT water extracts and metformin 250 mg/kg in comparison to those of HFD control mice, dose-dependently. Especially, GNYJT water extracts 200 mg/kg showed similar inhibitory activities against HFD-induced hepatic GK activity decreases in comparison to metformin 250 mg/kg (Table 5).
Table 5
Changes on the Hepatic Glucose-regulating Enzyme Activities in NFD or HFD Supplied Mice
6. Effects on lipid metabolism-related gene expressions
1) Effects on the hepatic AMPKα1, AMPKα2 and ACC1 mRNA expressions
The increases of hepatic ACC1 mRNA expressions, and decreases of AMPKα1 and AMPKα2 mRNA expressions were significantly (p<0.01 or p<0.05) regulated by administration of all three different dosages of GNYJT water extracts and metformin 250 mg/kg in comparison to those of HFD control mice, dose-dependently. Especially, GNYJT water extracts 200 mg/kg showed similar inhibitory activities against HFD-induced abnormal hepatic ACC1, AMPKα1 and AMPKα2 mRNA expression changes in comparison to metformin 250 mg/kg, in the current realtime RT-PCR analysis (Table 6).
Table 6
Changes on Lipid Metabolism-related Gene mRNA Expressions in Liver of NFD or HFD Supplied Mice in NFD or HFD Supplied Mice
2) Effects on the adipose tissue lipid metabolism -related gene mRNA expressions
The increases of adipose tissue leptin, C/EBPα, C/EBPβ, FAS, SREBP1c and PPARγ mRNA expressions, and decreases of UCP2, adiponectin and PPARα mRNA expressions were significantly (p<0.01 or p<0.05) restored by treatment of all three different dosages of GNYJT water extracts and metformin 250 mg/kg in comparison to those of HFD control mice, dose-dependently. Especially, GNYJT water extracts 200 mg/kg showed similar inhibitory activities against HFD-induced abnormal adipose tissue leptin, C/EBPα, C/EBPβ, FAS, SREBP1, PPARγ, UCP2, adiponectin and PPARα mRNA expression changes in comparison to metformin 250 mg/kg, in realtime RT-PCR analysis (Table 7).
Table 7
Changes on Lipid Metabolism-related Gene mRNA Expressions in Adipose Tissue of NFD or HFD Supplied Mice, Realtime RT-PCR Analysis
IV. Discussion
Despite the dramatic increase in prevalence of diabetes, dyslipidemia, obesity and related complications, patients have no choice but to take multiple drugs, as no effective multi-targeted treatment has been developed. As various adverse effects of existing drugs26 and risk of polypharmacy27 had been reported, effective preventive and therapeutic agent to aim multiple diseases has been on demand.
Since inhibition of oxidative stress and control of postprandial hyperglycemia are recommended to be essential in the treatment of diabetes28, many attempts had been made for searching safe and effective antioxidants and α-glucosidase inhibitors from natural substances to develop lead compounds for curing diabetes and related complications14. Although several drugs for their management have been suggested, none of them have shown noticeable efficacy in extensive spectrum of diabetes and related complications. Also, there are a number of limitations, such as high rates of secondary failure and various adverse effects7, so lifestyle interventions including weight loss and exercise are the only confirmed therapies for this disease. However, these are often challenging for sufferers to maintain. Therefore, discovery of agents targeting elevated hepatic lipid, glucose and oxidative stress that are safe for long-term administration are urgently required. Especially, Korean traditional medicines are characterized by global regulation of glucose, lipid metabolism and energy homeostasis via multiple ingredients in a prescription. Therefore, it is valuable to explore novel drugs with less negative effects from herbal extracts than synthetic antidiabetic drugs for prevention and possible treatment of diabetes and related complications.
Yijin-tang (二陳湯, YJT) is introduced in “Tai Ping Era (Tai Ping Hui Min He Ji Ju Fang, 1078)”29 written by Shiwen Chen, and then revised by Heo Jun at 1613, in Korea. Heo Jun described YJT as fundamental treatment of phlegm patterns in “Donguibogam”. It is composed of Citri Unshius Pericarpium, Pinelliae Tuber, Poria Sclerotium, Glycyrrhizae Radix et Rhizoma, and Zingiberis Rhizoma Recens. Several studies have insisted positive effect of YJT9,30-33 on treatment of diabetes and related complications.
In this study, GNYJT water extracts have demonstrated ameliorating effects on obesity, diabetes, dyslipidemia and other related complications. Although the decrease of mean daily food consumption was detected in HFD control mice in comparison to NFD supplied intact mice, it was not considered as critical problems in this study because the energy of HFD (4.73 kcal/g) used in the present study were relatively higher than that of NFD (4.00 kcal/g). In HFD supplied mice, satisfactory reductions of daily food consumption have already been reported in previous studies8,12,14,15,22,34. In the current study, no significant or meaningful difference on the average daily food consumption were observed in all treated groups, involving 250 mg/kg metformin in comparison to HFD control group. It has advocated that pharmacological effects of GNYJT water extracts monitored in this study are difficult to be considered as the results of simple food consumption disturbance, at least in a condition of current experiment.
They significantly inhibited HFD induced increase of body weight, body weight gain, fatty acid accumulation and adipocyte hypertrophy. Cellular hypertrophy and adipose tissue accumulation are major characteristic of intra-abdominal adipose tissue proliferation in rodents8,12,14,15,22,34 and obesity35, respectively. Excessive fatty acid intake result in increased TG accumulation in various tissues and lipolysis in adipose tissue. Resultingly, elevated free fatty acid (FFA) circulation provokes excessive fatty acid accumulation in non-adipose tissues, such as liver, and muscle.
Also, changes in the secretion, expression, and action of adipose tissue secreted adipokines are associated with various diseases, involving insulin resistance8,12,14,36. In insulin resistant situation, elevated levels of fatty acid-binding and transporting proteins promote the FFA uptake processes in non-adipose tissues. Especially in muscle, fatty acid accumulation has adverse effects in insulin-mediated muscular insulin signaling and glucose utilization. In addition, prolonged exposure to FFA in pancreas might cause insulin release impairment, which attributes to lipotoxicity37. High level of hepatic FFA concentration provokes insulin resistance, and elevates glucose release from liver38. Hyperglycemia is the main signal of diabetes, and its management is required to treat diabetes8,12,14,15. HFD fed mice have been applied as type II diabetic animal model, which had shown noticeable hyperglycemia8,12,13,15,22,34. Besides, with progression of HFD induced insulin-resistance, remarkable elevations of serum Hb1Ac and insulin levels have been observed8,12. HbA1c is a type of hemoglobin that is produced by high glucose overexposed erythrocytes, and is chiefly measured to estimate the mean serum glucose concentration over extended periods39.
As diabetes progressed chronically in HFD mice, dyslipidemia has also been generally accompanied40. Because the most critical problem of dyslipidemia is the elevation of serum LDL, TG and TC levels with decrease of HDL levels8,12,13,15, the efficacy of hypolipidemic agents was frequently evaluated by changes of these levels8,12,14,15,22,34. TG accumulation in liver also induces non-alcoholic fatty liver disease (NAFLD). It puts damage on liver, which has a key role in glucose metabolism, and resultingly leading to steatosis, steatohepatitis, hepatocellular necrosis or fibrosis41. Therefore, maintaining the balance between lipolysis and hepatic lipogenesis is essential in amelioration of insulin resistance and NAFLD.
Regulation of hepatic glucose-regulating enzyme activities were also demonstrated by GNYJT treatment. The hepatic enzyme GK is involved in glucose homeostasis, and it accelerates promotion of serum glucose use for production of energy or hepatic glycogen storage, bringing a decrease in the serum glucose level42. On the other hand, glucose-6-phosphatase (G6pase) and PEPCK are related to hepatic glucose output and gluconeogenesis, and take part in increasing serum glucose level43,44. In general, noticeable reduction of hepatic GK activity with facilitation of PEPCK and G6pase activities have been induced by HFD supply8,12, and so were in HFD control group of current study.
To elucidate the mechanisms of GNYJT in exhibiting ameliorating effects on diabetes and related complications, this experiment investigated gene mRNA expressions related to lipid metabolism in the hepatic and adipose tissues. PPAR and AMPK are major cellular regulator of lipid and glucose metabolism45, which plays a vital role in mediating hepatic lipogenesis46. AMPK exerts regulation of glucose and lipid metabolism through stimulation of fatty acid oxidation, lipogenesis inhibition and glucose production45. Recent studies have shown that AMPK activity is suppressed by the elements implicated in the occurrence of NAFLD, such as obesity and inflammation47. It is also well documented that PPARα exerts a pivotal role in the activation of beta-oxidation, and PPARγ take a role in the activation of tissue lipogenesis20. Therefore, inhibition of lipogenesis by AMPK and PPARα activation, with activation of lipid oxidation by down regulation of PPARγ, are suggested as a feasible therapeutic method to prevent the development of obese diabetes and related complications including NAFLD20,48,49. UCP2 is a representative thermogenesis-related protein50, and the effect of adiponectin on fatty-acid-oxidation and insulin sensitization depends on AMPK in adipose tissue and liver51. Additionally, acceleration of AMPK signaling pathway and facilitation of AMPK has shown substantial regulation on endogenous antioxidant defense systems44,65 and glucose-regulatory enzyme activities45. Therefore, this study investigated whether GNYJT water extracts affect AMPK signaling pathway related proteins and AMPK mRNA expression, in liver and adipose tissues.
Overall, oral administration of all three different dosages of GNYJT water extracts and metformin 250 mg/kg effectively demonstrated dose-dependent antiobesic, antidiabetic, anti-hyperlipidemic, hepato-protective, anti-oxidative effects and regulations of hepatic glucose-regulating enzyme activities and lipid metabolism-related gene expressions. Previously, some herbal medicines as preventive and therapeutic agents for diabetes and related complications had been experimented, with its effects compared to metformin. Zhenqing Recipe (ZQR) 3.78 g/kg/day was observed with its antidiabetic and hypolipidemic effects, and it came out to have comparable effect with metformin 150 mg/kg/day52. It reduced body and liver weight, serum glucose, TG, TC, insulin and hepatic TG, and improved hepatic steatosis. It also modulated glucose metabolism by reducing PEPCK, G6pase and CREB-regulated transcription coactivator 2 (CRTC2) proteins, and regulating Salt Inducible Kinase 1 (SIK1)/CRTC2 signaling pathway. In other study, Jinlida (JLD) 3000 mg/kg/day showed comparable effects with metformin 200 mg/kg/day53. It has regulated body weight, serum glucose, HbA1c, insulin, lipid levels. Also, modulation of insulin resistance, insulin signaling pathway (Insulin Receptor (INSR), IRS-1, Protein kinase B (AKT), glucose transporter 2 (GLUT2) and Phosphoinositide 3-kinase (PI3K)), and antioxidant effects (shown by regulation of reactive oxygen species (ROS), catalase (CAT), superoxide dismutase (SOD) and glutathione (GSH) levels) were observed. In addition, there were 3 in vivo studies demonstrating antiobesic, antidiabetic or hypolipidemic effects yijin-tang water extract (YJT, Erchen decotion), similarly demonstrating regulation of body weights, abdominal circumference, serum lipid levels, adipocyte diameter and insulin sensitivity. YJT 4350 mg/kg was compared with atorvastatin 10 mg/kg54 and YJT 8700 mg/kg/day with simvastatin 2 mg/kg9. Also, YJT 2280, 4570, 9140 mg/kg/day administered mice were compared with diabetic mice32. As current study demonstrated comparable effect of 200 mg/kg/day GNYJT and 250 mg/kg/day metformin, GNYJT water extract may demonstrate preventive and ameliorating effect in lower dose. Additionally, metabolic syndrome is defined by coincidence of hyperglycemia, central obesity, hypertriglyceridemia, low HDL-C level or hypertension55. As our diabetic-obese mice had displayed four aspects of metabolic syndrome except for hypertension, and GNYJT water extract has demonstrated antiobesic, antidiabetic and antidyslipidemic effects, we could possibly indicate potentials of GNYJT, to be applied in amelioration of metabolic syndrome.
V. Conclusion
This study demonstrated the antiobesic, antidiabetic and hypolipidemic effects of GNYJT water extract on obese-diabetic mice, through glucose and lipid metabolism regulating effects.
1. All three doses of GNYJT dose-dependently and significantly decreased body weight, total and abdominal fat mass, abdominal and periovarian fat pad weights, thickness and adipocyte diameters, demonstrating antiobesic effects.
2. All three doses of GNYJT dose-dependently and significantly reduced serum glucose, insulin, HbA1c levels and HOMA-IR demonstrating antidiabetic effects.
3. All three doses of GNYJT dose-dependently and significantly decreased reduced serum TC, TG, LDL levels and increased serum HDL level. Also, fecal TC and TG contents were significantly and dose-dependently increased by all three doses of GNYJT, demonstrating hypolipidemic effects.
4. All three doses of GNYJT dose-dependently and significantly decreased liver weights, liver steatosis and hepatocyte diameter, demonstrating hepatoprotective effects.
5. All three doses of GNYJT dose-dependently and significantly increased hepatic GK activity, and decreased G6pase, PEPCK activity, which demonstrated regulation of glucose metabolism.
6. All three doses of GNYJT dose-dependently and significantly down-regulated the mRNA expressions of hepatic ACC1, AMPKα1 and AMPKα2, decreased adipose tissue leptin, C/EBPα, C/EBPβ, FAS, SREBP1c and PPARγ mRNA expressions and increased UCP2, adiponectin and PPARα mRNA expressions, demonstrating regulation of lipid metabolism.











