Lee, An, Kim, and Baek: Potentials of Chenpi on Metabolic Syndrome: A Review
Abstract
Objectives
Metabolic Syndrome (MetS) is strongly related with central obesity, hypertriglyceridemia, low high-density lipoprotein-cholesterol (HDL-C), hyperglycemia, and hypertension. This study reviewed the potential of Chenpi in treatment of MetS through amelioration of co-related diseases, such as diabetes mellitus, hyperlipidemia, obesity, hepatic steatosis, and inflammation.
Methods
Six electronic databases (Oriental Medicine Advanced Searching Integrated System (OASIS), Korean Traditional Knowledge Portal, Korea Institute of Oriental Medicine (KIOM), Research Information Sharing Service (RISS), PubMed, and Embase) were used to search for in vitro, in vivo, and clinical research that discusses the potential effects of Chenpi (Citrus unshiu Markovich, Citrus reticulata Blanco) on diabetes mellitus, hyperlipidemia, obesity, hepatic steatosis, and inflammation.
Results
This review suggests the potential of Chenpi as a candidate for the treatment of metabolic syndrome through improvement of co-related diabetes, hyperlipidemia, obesity, hepatic steatosis, and inflammation. However, comparison of the results of each study was limited by a lack of quantification of the experimental materials.
KeywordsKeywords: Chenpi, Citrus unshiu peel, Citrus reticulata Blanco peel, metabolic syndrome, diabetes
I. Introduction
Metabolic syndrome (MetS), also named as syndrome X, can be diagnosed when more than three symptoms exist out of following five pathological conditions ; central obesity, hypertriglyceridemia, low high density lipoprotein-cholesterol (HDL-c), hyperglycemia or hypertension (HTN) 1. As sedentary lifestyle and over-nutrition became more common in many developed countries, the MetS has become epidemic 2. The global prevalence of MetS is hard to measure, but it can be estimated by the incidence of obesity and type 2 diabetes (T2DM). Years between 1998 and 2007, the prevalence of MetS in Korean population had recorded 31.3%, and the most common pathological condition was dyslipidemia 3. On 2012, the domestic prevalence of MetS recorded 28.8% for population over 30 years old, based on 2007-2010 National Health and Nutrition Survey 4. The diagnose of MetS leads to increased episodes of cardiovascular diseases (CVD), including stroke and myocardial infarction 5. The risk factors that would provoke vulnerability to CVD include age, gender, family history of CVD, T2DM, insulin resistance, hyperlipidemia (HL), HTN, obesity and non-alcoholic fatty liver (NAFLD) 6. Owing to multiple pathologic conditions, medicines targeting each of them are prescribed, such as hypoglycemic agents, lipid-lowering agents, antihypertensive agents, anti-platelet drugs, and weight-loss agents 7. Increased risks of atherosclerotic cardiovascular disease (ASCVD) are consequences of cholesterol level imbalance 8. Statins are used to reduce low density lipoprotein cholesterol (LDL-C) and total cholesterol levels, but adverse effects including musculoskeletal pain, gastrointestinal events, headache, and elevation of blood glucose and glycohemoglobin levels are present 9,10. Also, as CVD is the most common risk factor of mortality in diabetes, biguanide (metformin) is commonly prescribed to MetS patients with T2DM 11. However, biguanides may induce vitamin B12 deficiency, gastrointestinal disease, hemolytic anemia, hyperlactatemia, and metabolic acidosis 12. Therefore, many attempts have been done to find effective therapy of MetS with less side effects. With growing demand for natural products in MetS treatment, there had been several herbal agents reviewed about their MetS modulating effects. Some of the herbs include Satureja Species 13, sylimarin 14, Saffron 15and Ginger 16. Chenpi, the peel of Citrus unshiu Markovich (CMP) or Citrus reticulata Blanco (CBP), has traditionally been used to treat indigestion, improve bronchial conditions, and blood circulation in east Asia 17. It contains various phytochemicals such as hesperidin, naringin and nobiletin 18. Among them, hesperidin, which has largest proportion, has been proven to have anti-inflammatory 19, neuroprotective 20, anti-cancer 21, anti-diabetic, antihypertensive and antioxidant and CVD risk lowering effects 22. Although several reviews about each single flavonoids are present, there are none about the herbal plant itself. As Chenpi is comprised of various phytochemicals, the effect should be differentiated from that of single constituents. In aspects of MetS, diverse values of Chenpi have been discovered until now. These values are summarized as follows; 1. Anti-diabetic effect, 2. Lipid-lowering effect, 3. Anti-obesity effect, 4. Anti-hepatic steatosis activity, and 5. Anti-inflammatory effect.
II. Method
A. Selection of database and searching method
The purpose of this review is to discuss the effects of Chenpi on different elements of MetS, T2DM, HL, obesity and HTN, through analyzing human and experimental papers. Literature published from 2011 to date, in language of Korean and English were included. The database used for searching the literatures were Oriental Medicine Advanced Searching Integrated System (OASIS, https://oasis.kiom.re.kr/), Korean Traditional Knowledge Portal ( https://www. koreantk.com/ktkp2014/), Korea Institute of Oriental Medicine (KIOM, https:// www.kiom.re.kr/), Research Information Sharing Service (RISS, http://www. riss.kr) to search for Korean literature, and PubMed ( https://www.ncbi.nlm.nih.gov/pubmed), Embase ( https://www.embase.com) to search for western research papers. In domestic and English search, the keyword of ‘metabolic syndrome’, ‘syndrome X’, ‘diabetes’, ‘DM’, ‘diabetes mellitus’, ‘hyperglycemia’, ‘hyperlipidemia’, ‘dyslipidemia’, ‘lipid’, ‘obesity’, ‘NAFLD’, ‘fatty liver’, ‘hepatic steatosis’, ‘inflammation’, ‘Citrus unshiuMarkovich pericarpium’, ‘Citrus unshiuMarkovich peel’, ‘Citri reticulatae pericarpium’, ‘Citrus reticulata Blanco peel’, ‘Citrus reticulata blanco pericarpium’ were included. We combined these keywords regarding characteristic of each database. The searching procedure was done between 2021.07.01-2021.07.04.
B. Selection and exclusion
1. Selection criteria
a) In vitro/in vivo experimental study b) Clinical study on patients with hypertension, type 2 diabetes, hyperlipidemia, obesity, or hepatic steatosis c) Exclusive study on freeze-dried powder, water extract, ethanol extract or fermented product of Citrus unshiu Markovich peel or Citrus reticulata Blanco peel d) Study including measurements indicating treatment effects about hypertension, type 2 diabetes, hyperlipidemia, obesity, or hepatic steatosis e) Study written in Korean or English
2. Exclusion criteria
a) Study using combined herbal treatments b) Study not including measurements indicating treatment effects about hypertension, type 2 diabetes, hyperlipidemia, obesity, or hepatic steatosis
C. Material selection and study analysis
We collected literatures searched in domestic and English databases, and excluded duplicating literatures based on study title, published year and authors. First step of screening was conducted based on title and abstract, following selection and exclusion criteria. Second step of screening was conducted through examining full text of previous screened records. In sequence, clinical application possibility of Chenpi was judged, and the final literature selection and analysis was conducted.
D. Data extraction
On our study, full texts of finally selected literatures were analyzed. Data about study methods, experimental model(species, number), interventions of treatment and control group (including dose and duration), positive control, measurements and results were extracted. Based on extracted data, characteristics of each literature were discussed.
III. Result
A. Literature screening
At first, 85 literatures were searched, and 50 literatures were selected after exclusion of 35 duplicating literatures. On first step of screening, titles and abstracts were examined following selection and exclusion criteria, and 14 literatures which were not related to Chenpi and MetS were excluded. On second screening, investigation of full text removed 17 literatures using treatments combined with other herbs, flavonoids or written in other language than Korean or English. As a final outcome, 19 literatures were selected on our study ( Fig. 1).
Fig. 1
PRISMA flow diagram for process or literature research. 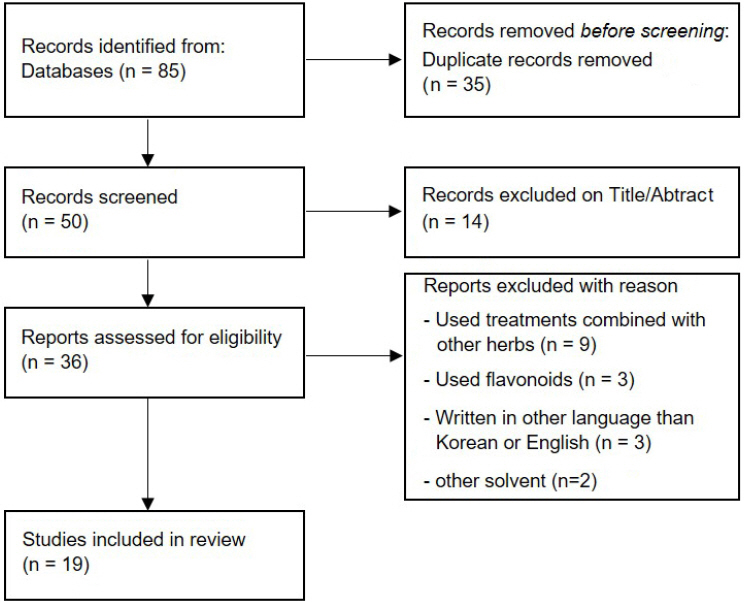
B. Literature analyzing
1. Characteristics of the selected literatures
Among 19 selected literatures, there were 7 23-29 in vitro, 8 30-37 in vivo, 2 38,39 in vitro and in vivo, and 2 40,41 clinical researches. 11 23,24,26-28,30,32,34,38,39,41 were published in Korea, 3 35-37 in United States, 3 27,29,40 in international journals, and 1 of each in Switzerland 33, India 25 and Japan 31.
2. Analyze of experimental models
Among 9 in vitro studies, RIN-m5f β-cells 38, HIT-15 cells 39, OP9 cells 23, HepG2 cells 29, Human embryonic kidney 293 cells 25 and NIT-1(murine pancreatic β-cells) 25 were used in 1 of each studies. L6 myotubes in were used in 2 studies 25,38 and 3T3-L1 cells were used in 3 studies 24,27,39. Among 10 in vivo studies, C57BL/KsJ-db/db mice 35 and Wistar-Hannover GALAS rats 31 were used in 1 of each study. 3 studies 28,36,38 selected C57BL/6 mice and 6 studies 30,32-34,37,39 selected Sprague Dawely rats. In 2 clinical researches, patient with hypertriglyceridemia 41 and obesity (BMI>23 kg/m2) 40 were chosen.
3. Analyze of interventions
16 literatures 23-35,39-41 selected Citrus unshiu Markovich peel, and 3 36-38 selected Citrus reticulata Blanco peel as an intervention. There were various extraction methods of Chenpi. 4 literatures 23,26,37,38 used water extraction, 10 24,25,28,32-36,39,41 used ethanol extraction, and 2 27,29 chose both water and ethanol extraction. Chenpi powder 30, Chenpi juice extract 40 and 1% albedo TDF extracted by method of Prosky 31 were also chosen. Furthermore, 6 literatures contained positive control groups. Rosiglitazone 0.001 g/100 g diet 35, estradiol (E2) 10 μg/kg 33, sinetrol 0-0.5 mg/mL 27,37, simvastatin 1.04 mg/kg 37, resveratrol 0.1% 36 and insulin 1 μM 26 were used. C. Results
1. Diabetes Mellitus
Diabetes is a worldwide health problem, with the estimated prevalence of 6.4%, globally. The total predicted increase in prevalence of T2DM from 2010 to 2030 is 54%, with estimated annual growth of 2.2%, which almost doubles the current annual growth of the global adult population 42. Insulin resistance (IR) is frequently related to central obesity and CVD. Accordingly, visceral adipocytes secrete adipokines, which are the inflammatory cytokines, and they are thought to be closely concerned with the pathological conditions of MetS 43. The effect of Chenpi on DM is analyzed below ( Table 1).
Table 1
Summary of studies on Chenpi in Diabetes Mellitus
|
Reference and year |
Method |
Dose and duration |
Experimental model |
Control and intervention groups |
Positive control |
Effect |
|
Park, 201139
|
in vivo |
FCMPE and CMPE, each 0.1% or 0.5% of total diet, for 10 weeks |
HFD Sprague
Dawley rat |
1) Normal diet (n=8)
2) HFD (n=8)
3) HFD+CMPE 0.1% (n=8)
4) HFD+CMPE 0.5% (n=8)
5) HFD+FCMPE 0.1% (n=8)
6) HFD+FCMPE 0.5% (n=8) |
none |
↓Serum glucose level in FCMPE 0.1%, 0.5% and CMPE 0.5% group (p<0.05) |
|
in vitro |
FCMPE and CMPE 0.01, 0.025 or 0.1 mg/mL |
Deoxyribose processed HIT-T15 cell |
1) Normal
2) Deoxyribose control
3) Deoxyribose+CMPE 0.01 mg/mL
4) Deoxyribose+CMPE 0.025 mg/mL
5) Deoxyribose+CMPE 0.1 mg/mL
6) Deoxyribose+FCMPE 0.01 mg/mL
7) Deoxyribose+FCMPE 0.025 mg/mL
8) Deoxyribose+FCMPE 0.1 mg/mL |
none |
↑Cell viability in all groups
↑Insulin secretion in all groups |
|
Park, 201335
|
in vivo |
CMPE 2 g per 100 g diet for 6 weeks |
C57BL/
KsJ-db/db mice |
1) Control (n=10)
2) CMPE 2 g/100 g diet (n=10)
3) Rosiglitazone 0.001 g/100 g diet (n=10) |
rosiglitazone (0.001 g/
100 g diet) |
↓Fasting glucose level (p<0.05)
↓Hepatic glycogen content (p<0.05)
↑Serum insulin/glucagon ratio (p<0.05)
↓Activity of hepatic PEPCK (p<0.05) |
|
Park, 201728
|
in vivo |
CMPE 1%, FCMPE 0.3% or FCMPE 1% for 13 weeks (ad libitum) |
HFD
C57BL/6J mice |
1) LFD (n=8)
2) HFD (n=8)
3) HFD+CMPE 1% (n=8)
4) HFD+FCMPE 0.3% (n=8)
5) HFD+FCMPE 1% (n=8) |
none |
↓ Fasting glucose level in FCMPE 0.3% and 1% group (p<0.05)
↓AUC of IPGTT in FCMPE 0.3% group (p<0.05)
↑mRNA level of Gk in FCMPE 0.3% and 1% group (p<0.05)
↑mRNA level of Glut2 in FCMPE 1% group (p<0.05)
↓mRNA level of G6pase in CMPE 1% group (p<0.05) |
|
Kim, 201826
|
In vitro |
CMPW 50, 100, 200, 500 μg/ml |
L6 myoblast cells |
1) Control
2) Insulin 1 μM
3) CMPW 50 μg/ml
4) CMPW 100 μg/ml
5) CMPW 200 μg/ml
6) CMPW 500 μg/ml |
Insulin
1 μM |
↑IRS-1, PIK3R and GLUT4
mRNA expression in all groups, dose-dependently
↑Akt mRNA expression in CMPW 100, 200 and 500 μg/ml groups, dose-dependently |
|
CMPW 100, 500 μg/ml |
1) Control
2) Insulin 1 μM
3) CMPW 100 μg/ml
4) CMPW 500 μg/ml |
Insulin
1 μM |
↑Expression of Akt western blotting protein in 100 and 500 μg/ml group (p<0.001) |
|
Kim, 201925
|
In vitro |
CMPE 100, 200, 500 μg/mL |
Human embryonic kidney 293 cells |
1) Control
2) CMPE 100 μg/mL
3) CMPE 200 μg/mL
4) CMPE 500 μg/mL |
none |
↓Kv2.1 channel currents, but had no effect on Kv2.2 channel currents |
|
CMPE |
NIT-1 (murine pancreatic β-cell) |
1) Control
2) Rehmanniae Radix ethanol extract
3) Poria cocos Wolfethanol extract
4) CMPE |
none |
↑insulin secretion from NIT-1 cells (p<0.01) |
|
Ke, 202036
|
in vivo |
CBPE 0.2% or 0.5%, for 10 weeks |
HFD
C57BL/6J mice |
1) ND (n=8)
2) HFD (n=8)
3) HFD+0.1% resveratrol (n=8)
4) HFD+0.2% CBPE (n=8)
5) HFD+0.5% CBPE (n=8) |
0.1% resveratrol |
↓AUC value of GTT in both groups (p<0.05) |
|
Kwak, 202038
|
in vitro |
CMPW 100/200/300 μg/mL |
15 mM glucose processed RIN-m5f β-cell |
1) Normal control
2) Glucose
3) Glucose+CMPW 100 μg/mL
4) Glucose+CMPW 200 μg/mL
5) Glucose+CMPW 300 μg/mL |
none |
↑Insulin secretion in β cell (p<0.05) |
|
L6 myotubes |
1) Normal control
2) Insulin
3) Insulin+CMPW 100 μg/mL
4) Insulin+CMPW 200 μg/mL
5) Insulin+CMPW 300 μg/mL |
Insulin 10 nM |
↑Glucose uptake in myotubes (p<0.05) |
|
In vivo |
CMPW 200/300 mg/kg for 4 weeks |
diabetic C57BL/ 6 mice induced with STZ |
1) Diabetic mice (n=6)
2) Diabetic mice+CMPW 200 mg/kg (n=6)
3) Diabetic mice+CMPW 300 mg/kg (n=6) |
none |
↓Fasting blood glucose level in
both groups (p<0.05).
↓HbA1c level in both groups (p<0.05).
↑Serum insulin level in
both groups (p<0.05).
↓liver and kidney size in both groups
↓liver and kidney weight in both groups (p<0.05) |
|
in vivo |
CMPW 100/200/300 mg/kg |
C57BL/6 mice |
1) Normal control (n=6)
2) Acarbose 300 mg/kg (n=6)
3) CMPW 100 mg/kg (n=6)
4) CMPW 200 mg/kg (n=6)
5) CMPW 300 mg/kg (n=6) |
acarbose 300 mg/kg |
↓OSTT (p<0.05) |
|
CMPW 100/200/300 mg/kg |
C57BL/6 mice |
1) Normal control (n=6)
2) Metformin 200 mg/kg (n=6)
3) CMPW 100 mg/kg (n=6)
4) CMPW 200 mg/kg (n=6)
5) CMPW 300 mg/kg (n=6) |
metformin 200 mg/kg |
↓OGTT (p<0.05) |
a) Glucose-lowering effect
(a.1) In vivo
A number of in vivo studies have shown that Chenpi has glucose-lowering effect. Fasting blood glucose (FBS) level was significantly reduced by CMP ethanol extract (CMPE) 2 g per 100 g diet administered to diabetic mice (p<0.05) 35 CMP water extract (CMPW) 200 or 300 mg/kg to STZ induced diabetic mice (p<0.05) 38. They had tendency of being more effective dose-dependently. Bioconversed Chenpi was also studied for its anti-diabetic effects. Ethanol extract of CMP fermented by Aspergillus niger (FCMPE) 0.3%, 1% and CMPE 1% were fed ad libitum to HFD mice, and FBS was significantly lowered only in FCMPE groups (p<0.05) 28. Also, FCMPE bioconversed by Aureobasidium pullulans and CMPE 0.1% or 0.5% applied to HFD rats and reduction of serum glucose was observed in all FCMPE groups and 0.5% CMPE group (p<0.05) 39. By fermentation, naringin was bioconversed into naringenin. CMP water extract (CMPW) had glucose reducing effect, as well. Even though it had no significant effect in α-glucosidase inhibition, CMPW 100, 200 and 300 mg/kg administration significantly lowered glucose level in OSTT and OGTT (p<0.05). This result suggests the possibility of other hypoglycemic pathway existence, excluding α-glucosidase inhibition. b) Insulin-stimulating effect
(b.1) In vitro
Insulin secretion stimulating effect was also tested. In vitro studies revealed insulin-promoting effect of Chenpi. CMPW 100, 200 and 300 mg/kg was applied to 15 mM glucose processed RIN-m5f β-cells, and elevation of insulin secretion in β cells was monitored (p<0.05) 38. Aureobasidium pullulans bioconversed FCMPE and CMPE 0.01, 0.025 and 0.1 mg/mL applied to deoxyribose processed Hamster Islet Transformed-Tioguanine resistant clone 15 cell (HIT-T15 cell, pancreatic beta cell) stimulated insulin secretion in all groups 39. In this study, FCMPE performed better effect than CMPE, especially in high dose. General voltage-dependent K+ (Kv) channels has been proved to improve insulin secretion, membrane depolarization and Ca2+ influx in a glucose-dependent manner 44-46. Among them, Kv2.1 and Kv2.2 are enriched in the pancreatic islets, and Kv2.1 is enhanced in pancreatic β-cells 47. Kv2.1 modulates insulin secretion by affecting β-cells, and Kv2.2 regulates somatostatin release by affecting δ-cells. CMPE 100, 200, 500 μg/mL application to Human embryonic kidney 293 cells 25 inhibited Kv2.1 channel currents in a dose-dependent manner. However, none of them had effect on Kv2.2 channel current. Also, CMPE application to NIT-1 (murine pancreatic β-cell) resulted in significant increase of insulin secretion (p<0.01). Therefore, selective inhibition of Kv2.1 without cross-inhibition of Kv2.2 would exhibit increase of insulin secretion without concern of adverse effects. (b.2) In vivo
CMPE 2 g per 100 g diet fed to diabetic mice exhibited increase in serum insulin/glucagon ratio (p<0.05) 35. CMPW 200 and 300 mg/kg were fed to STZ induced diabetic mice, and serum insulin was on the rise in both groups, dose-dependently (p<0.05) 38. c) Glucose-regulating effect
(c.1) In vitro
In vitro study of CMPW 100, 200 and 300 mg/kg applied to L6 myotubes performed elevation of glucose uptake in myotubes (p<0.05) 38. (c.2) In vivo
In vivo study of CMPE 2 g per 100 g diet administration to diabetic mice demonstrated reduction of hepatic glycogen content (p<0.05) 35. d) Glycosylated Hemoglobin, Type A1C (HbA1c) reducing effect
(d.1) In vivo
CMPW 200 and 300 mg/kg were fed to STZ induced diabetic mice, and it resulted in decrease of HbA1c index (p<0.05).
e) Amelioration of glucose tolerance
(e.1) In vivo
In HFD mice, CBP ethanol extract (CBPE) 0.2% and 0.5% induced decrease in Area Under the Receiver Operating Characteristic Curve (AUC) value of glucose tolerance test (GTT) (p<0.05) in both groups 36. On a study conducted in 2017, fermented Chenpi showed decrease in AUC of Intraperitoneal Glucose Tolerance Test (IPGTT) 28. Among them, only 0.3% FCMPE has significant effect, suggesting that fermented Chenpi has better effect than normal Chenpi, and it’s efficient in low dose. f) Inhibition of gluconeogenesis
(f.1) In vitro
Protein kinase B (Akt) is one of signaling pathways that increases glucose uptake in cells. When insulin combines to insulin receptor, phosphorylation of insulin receptor substrate-1 (IRS-1) and activation of phosphatidylinositol 3-kinase regulatory (PI3KR) results in phosphorylation of Akt. Phosphorylated Akt transports glucose into intracellular space, by bumping transporter 4 (GLUT4) to membrane cell 48. As well, GLUT4 is a glucose transporter that is activated by insulin, and related influx of glucose into cells 49. In L6 myotubes, CMPW 50, 100, 200, 500 μg/ml up-regulated IRS-1, PIK3R and GLUT4 mRNA expression in all groups, dose-dependently. Akt mRNA expression in CMPW 100, 200 and 500 μg/ml groups were also increased, dose-dependently 26. (f.2) In vivo
There are several experiments about mRNA expression regulation related to glucose metabolism. Hepatic phosphoenol pyruvate carboxykinase (PEPCK) is an enzyme related to hepatic gluconeogenesis, which is activated in diabetic state, leading to insulin resistance 50. CMPE 2 g per 100 g diet applied to diabetic mice suppressed activity of hepatic PEPCK 35. Another study revealed activation of glucokinase (Gk), glucose transporter protein type 2 (Glut2) and suppression of glucose6phosphotase (G6pase) by Chenpi and fermented Chenpi medication to HFD mice 28. Gk phosphorylates glucose to glucose6phosphate, and its activation leads to glycolysis and glycogen synthesis. Glut2 is an enzyme found in liver that transports glucose and G6pase is a gluconeogenic enzyme. Up-regulation of GK and Glut2 mRNA expression were superior in FCMPE groups, and down-regulation of G6pase mRNA expression was superior in CMPE group (p<0.05). Regulation of these mRNA expressions implies anti-diabetic effect of Chenpi, presenting similar property of all types of Chenpi. g) Pancreatic beta cell protecting effect
(g.1) In vitro
Administration of FCMPE and CMPE 0.01, 0.025 and 0.1 mg/mL to deoxyribose processed HIT-T15 cell increased cell viability in all groups 39. h) Ameliorating effects in DM complication
(h.1) In vitro
In vitro study about CMPW 200 and 300 mg/kg administration to STZ induced diabetic mice reduced liver and kidney size, and lowered weight of them (p<0.05) in both groups 38. It suggests that Chenpi ameliorates DM complications.
2. Hyperlipidemia
The increase of apo B-containing lipoproteins is a major risk factor of coronary artery disease 51. The effect of Chenpi on hyperlipidemia (HL) is analyzed below ( Table 2).
Table 2
Summary of studies on Chenpi in Hyperlipidemia
|
Reference and year |
Method |
Dose and duration |
Experimental model |
Control and intervention groups |
Positive control |
Effect |
|
Park, 201134
|
in vivo |
CMPE
48.6 mg/kg (0.1%)
or 241.9 mg/kg (0.5%) for 10 weeks |
HFD Sprague Dawley rat |
1) ND (n=8)
2) HFD (n=8)
3) HFD+CMPE 0.1% (n=8)
4) HFD+CMPE 0.5% (n=8) |
none |
↓Serum TC, LDL-C level in all groups (p<0.05)
↓Serum TG level in 0.5% group (p<0.05)
↓AI*in 0.5% group (p<0.05)
↑Serum HDL-C level (p<0.05) |
|
Lee, 201141
|
clinical research |
CMPE 1,200 mg for 8 weeks |
91 patients with 170 mg/dL
-250 mg/dL of TC level |
1) Placebo (n=46)
2) CMPE 1200 mg (n=45) |
none |
↓Serum TG level (p<0.05)
↓Serum TC,
↑serum HDL-C level |
|
Park, 201139
|
in vivo |
FCMPE or CMPE, 0.1% or 0.5% of total diet, for 10 weeks |
HFD Sprague Dawley rat |
1) Normal diet (n=8)
2) HFD (n=8)
3) HFD+CMPE 0.1% (n=8)
4) HFD+CMPE 0.5% (n=8)
5) HFD+FCMPE 0.1% (n=8)
6) HFD+FCMPE 0.5% (n=8) |
none |
↓Serum TC level in all groups (p<0.05)
↓Serum LDL-C level in CMPE 0.1%, 0.5% and FCMPE 0.5% group (p<0.05)
↓Serum TG level in CMPE 0.5%, FCMPE 0.1% and 0.5% group (p<0.05)
↑Serum HDL-C level in CMPE 0.1%, 0.5% and FCMPE 0.5% group (p<0.05)
↓AI in CMPE 0.5%, FCMPE 0.1% and 0.5% group (p<0.05) |
|
Iwata, 201231
|
in vivo |
1% albedo TDF extracted by method of Prosky |
Wistar-Hannover GALAS rat |
1) Control (n=6)
2) 1% albedo TDF (n=6) |
none |
↓Serum TG level (p<0.05) |
|
Park, 201335
|
in vivo |
CMPE 2 g per 100 g diet for 6 weeks |
C57BL/KsJ-db/db mice |
1) Control (n=10)
2) CMPE 2 g/100 g diet (n=10)
3) Rosiglitazone 0.001 g/100 g diet (n=10) |
rosiglitazone (0.001 g/100 g diet) |
↓Serum TG level (p<0.05) |
|
Jung, 201332
|
in vivo |
CMPE 100 or 300 mg/kg. twice daily for 8 weeks. |
HFD Sprague Dawley rat |
1) ND (n=10)
2) HFD (n=10)
3) HFD+CMPE 100 mg/kg (n=10)
4) HFD+CMPE 300 mg/kg (n=10) |
none |
↓Serum TC level in 300 mg/kg group (p<0.001)
↓Serum TG level in 100 and 300 mg/kg group (p<0.05, p<0.001) |
|
Lim, 201433
|
in vivo |
CMPE 30, 100 or 300 mg/kg for 8 weeks |
OVX Sprague Dawley rat |
1) Sham (n=12)
2) OVX (n=12)
3) OVX+E2 10 μg/kg (n=12)
4) OVX+CMPE 30 mg/kg (n=12)
5) OVX+CMPE 100 mg/kg (n=12)
6) OVX+CMPE 300 mg/kg (n=12) |
E2 10 μg/kg |
↓Serum TC, TG, LDL-C level (p<0.05)
↑Serum HDL-C level (p<0.05)
↓CRI†, AIin 300 mg/kg group (p<0.01) |
|
Cho, 201430
|
in vivo |
CMP powder, 5%, 10% or 15%, for 4 weeks |
HFD Sprague Dawley rat |
1) ND
2) HFD
3) HFD+CMP 5%
4) HFD+CMP 10%
5) HFD+CMP 15% |
none |
↓Serum LDL-C level in
10% group (p<0.05)
↑Serum HDL-C level in
15% group (p<0.05)
↑HDL/TC ratio (p<0.05)
↑HDL/TC ratio (p<0.05)
↓AI in 10% and 15% group (p<0.05) |
|
Park, 201728
|
in vivo |
CMPE 1%, FCMPE 0.3% or FCMPE 1% for 13 weeks (ad libitum) |
HFD
C57BL/6J mice |
1) LFD (n=8)
2) HFD (n=8)
3) HFD+CMPE 1% (n=8)
4) HFD+FCMPE 0.3% (n=8)
5) HFD+FCMPE 1% (n=8) |
none |
↓Serum TC level in FCMPE 0.3% and 1% group (p<0.05)
↓Srebp1c level in FCMPE 0.3% and 1% group (p<0.05)
↓Fas level in FCMPE 0.3% group (p<0.05)
↓Acc, Cpt1 level in FCPE 1% group (p<0.05)
↓Srebp2, Hmgr, Pcsk9 level in FCPE 0.3% and 1% group (p<0.05) |
|
Kang, 201840
|
clinical research |
CMP juice extract 18 mg for 4 weeks |
118 adult patients.
BMI>23 kg/m2 |
1) 118 patients with BMI>23
(Female : 88, Male : 30) |
none |
↓Serum TC level (p<0.0001)
↓Serum LDL-C level (p=0.011) |
|
Ke, 202036
|
in vivo |
CBPE 0.2% or 0.5%, for 10 weeks |
HFD
C57BL/6 mice |
1) ND (n=8)
2) HFD (n=8)
3) HFD+0.1% resveratrol (n=8)
4) HFD+0.2% CBPE (n=8)
5) HFD+0.5% CBPE (n=8) |
0.1% resveratrol |
↓Serum LDL-C and TG level in both groups (p<0.05)
↓Serum TC level in
0.5% group (p<0.05) |
|
Zeng, 202037
|
in vivo |
CBPW 1.04 g/kg, for 4 weeks |
HFD
Sprague-Dawley rat |
1) ND (n=8)
2) HFD (n=8)
3) HFD+1.04 mg/kg simvastatin (n=8)
4) HFD+CBPW 1.04 g/kg (n=8) |
1.04 mg/kg simvastatin |
↓Serum TC, TG, LDL-C level (p<0.05)
↑Serum HDL-Clevel (p<0.05)
↓5-L-Glutamyl-taurinelevel (p<0.05)
↓Cis-4-octenedioic acid and 2-octenedioic acidlevel (p<0.05)
↓5-Aminopentanoic acidlevel (p<0.05) |
a) Serum triglyceride, cholesterol reducing effect
Low serum HDL and elevated serum TG level are convincing markers of cardiovascular diseases, and causes an increase of serum LDL level, resulting in increased cardiovascular risk 52. (a.1) In vivo
Various in vivo studies have been done to test hypolipidemic effects in HFD rats. Medication of CMPE 48.6 mg/kg (0.1%) and 241.9 mg/kg (0.5%) significantly reduced serum total cholesterol (TC), triglyceride (TG) and low density lipoprotein cholesterol (LDL-C) and elevated high density lipoprotein cholesterol (HDL-C) level (p<0.05) 34. Administration of Aureobasidium pullulans fermented CMPE and CMPE 0.1%, 0.5% induced reduction of serum TG, TC, LDL-C and elevation of HDL-C level (p<0.05) 39. Aspergillus niger fermented CMPE 0.3% and 1% reduced serum TC level (p<0.05) 28. CMPE 100 and 300 mg/kg were applied to HFD Sprague Dawley mice twice daily for 8 weeks 32. Serum TC level was significantly lowered in 300 mg/kg group (p<0.001), also serum TG level in 100 (p<0.05) and 300 (p<0.001) mg/kg group. CBPE 0.2% and 0.5% lowered serum TG, LDL-C level in both groups (p<0.05), and TC level in 0.5% group (p<0.05) 36. In 2020, 0.2% and 0.5% of CBPE were fed to HFD C57BL/6 mice, for 10 weeks 36. Resultingly, decrease of LDL-C and TG in both groups, and TC in 0.5% group were observed, all significantly (p<0.05). CBPW 1.04 g/kg reduced serum TG, TC, LDL-C level (p<0.05) and elevated HDL-C level (p<0.05) 37. As well, the efficacy of CMP powder in dyslipidemia was discovered 30. Freeze-dried CMP, each 5%, 10%, 15% of HFD diet, were fed to Sprague Dawley mice for 4 weeks. Serum LDL-C was reduced in 10% group (p<0.05) and serum HDL-C was increased in 15% group (p<0.05). In both 10% and 15% groups, HDL/TC ratio was elevated, and AI was lowered, all significantly (p<0.05). In diabetic mice, CMPE 2 g per 100 g diet significantly lowered serum TG level (p<0.05), even though there were no change in plasma TC and free fatty acid level 35. In OVX rats, apply of CMPE 30, 100 and 300 mg/kg resulted in decrease of TG, TC, LDL-C level and increase of HDL-C level (p<0.05) 33. (a.2) Clinical research
Clinical research also has been done on patients with 170 mg/dL-250 mg/dL of TC level. CMPE 1200 mg medicated to 91 patients with 170 mg/dl -250 mg/dl of TC level resulted in significant reduction of serum TG level (p<0.05) and noticeable change in serum TC and HDL-C level 41. Besides, there was no evidence of hepatotoxicity, as no significant differences in serum GOT, GPT, γ-glutamyl transferase (ɣ-GT) level were noted between CMPE and placebo groups. CMP juice extract 18 mg lowered serum TC (p<0.0001) and LDL-C level (p=0.011) in 118 adult patients with body mass index (BMI) over 23 kg/m 240. The hypolipidemic effect of albedo, the white part of CMP, has also been tested. Albedo, which contains arabinose, galactose, xylose, and glucose, was extracted as total dietary fiber (TDF) by the method of Prosky. Wistar-Hannover GALAS rats were fed freely with diet containing 4% cellulose and 1% TDF, and their serum TG was significantly reduced (p<0.05) 31. b) Alleviation of coronary artery risk index and atherogenic index
(b.1) In vivo
Currently, atherogenic index (AI), a ratio of serum lipid concentrations, has been recommended as a biomarker for cardiovascular diseases and atherosclerosis 53-55. Coronary artery risk index (CRI), is also related with MetS 56. Four in vivo studies have shown significant alleviation of AI. CMPE 241.9 mg/kg (0.5%) 34, and CMP powder 10% 30 each led to significant reduction of AI (p<0.05) in HFD rats. In other study, Aureobasidium pullulans fermented Chenpi has been revealed to be more effective than non-fermented Chenpi in lowering AI. In HFD rats, FCMPE 0.1% and 0.5% and CMPE 0.5% of total diet significantly lowered AI (p<0.05) 39, showing that only FCMPE has significant risk-lowering effect in same dose (0.1% of total diet). Also, study about effect of Chenpi CRI and AI has been conducted. In OVX rats, CMPE 300 mg/kg significantly decreased CRI and AI (p<0.01) 33. OVX rat, a model of postmenopausal symptoms, has shown bone loss caused by estrogen deficiency and lipid metabolic disturbance. It is known that CMP extract has both plasma and hepatic lipid-lowering effect through inhibition of 3-Hydroxy-3-Methyl Glutaryl-Coenzyme A (HMG-CoA) reductase activity 57. The outcomes were reduction of TC, TG, LDL-C (p<0.05) and elevation of HDL-C (p<0.05) level. Also, coronary artery risk index (CRI) and atherogenic index (AI) were lowered (p<0.01). This study has confirmed risk lowering effect of CVDs, which are the major complications of MetS. c) Reduction of apolipoprotein B-100
(c.1) In vivo
Dyslipidemia is one of the common insulin resistance complications of metabolic syndrome. It is characterized by elevated atherogenic lipid and lipoprotein profile, especially hepatic very low-density lipoprotein (VLDL) overproduction. Elevated hepatic VLDL secretion leads to increased plasma apolipoprotein B100 (apoB-100)-containing lipoprotein.
d) Regulation of mRNA expressions related to serum plasma metabolism
(d.1) In vivo
Aspergillus niger fermented Chenpi also was discovered to have hypolipidemic effects by modulating mRNA expressions involved with lipid-metabolism. CMPE 1%, FCMPE 0.3% and FCMPE 1% were applied to HFD mice for 13 weeks 28. As a result, significant suppressions of those mRNA expressions were observed only in fermented Chenpi groups. Sterol regulatory element binding protein 1c (Srebp1c) mRNA expression in both FCMPE groups, fatty acid synthase (Fas) mRNA expression in 0.3% FCMPE group, and acetyl CoA carboxylase (Acc), carnitin palmitoyl transferase 1 (Cpt1) mRNA expression in 1% FCMPE group were all significantly reduced (p<0.05). These mRNAs are all related to hepatic lipogenesis and fatty acid oxidation. Also, expressions of sterol regulatory element binding protein 2 (Srebp2), HMG CoA reductase (Hmgr) and proprotein convertase subtilisin/kexin type 9 (Pcsk9), which are related to hepatic cholesterol homeostasis, were significantly reduced in FCMPE 0.3% and 1% group (p<0.05). e) Regulation of biomarkers related to plasma lipid metabolism
(e.1) In vivo
CPW reversed abnormal changes in biomarkers, related to aurine and hypotaurine metabolism, fatty acid biosynthesis and arginine and proline metabolism 37. 5-L-Glutamyl-taurine, an intermediate product of taurine metabolism which is related to oxidative stress reaction was significantly decreased after CPW treatment (p<0.05). Cis-4-Octenedioic acid and 2-octenedioic acid, the unsaturated fatty acids which increases in abnormal fatty acid metabolism situation, was also reduced by CPW (p<0.05). Additionally, 5-Aminopentanoic acid, a lysine degradation product, was decreased significantly (p<0.05).
3. Obesity
The overweight and obesity are the risk factors of HL, HTN, IR and T2DM 60. Worldwide overweight currency is estimated about 2 billion, and one-third of them satisfies the criteria of obesity 61. Since World Health Organization (WHO) has defined obesity as a global epidemic in 1996 62, the demand for effective treatments raised, as well as herbal therapy. The effect of Chenpi on obesity is analyzed below ( Table 3).
Table 3
Summary of studies on Chenpi in Obesity
|
Reference and year |
Method |
Dose and duration |
Experimental model |
Control and intervention groups |
Positive control |
Effect |
|
Park, 201134
|
in vivo |
CMPE 48.6 mg/kg (0.1%) or
241.9 mg/kg (0.5%) for 10 weeks |
HFD Sprague
Dawley rat |
1) ND (n=8)
2) HFD (n=8)
3) HFD+CMPE 0.1% (n=8)
4) HFD+CMPE 0.5% (n=8) |
none |
↓FER*in 0.5% group (p<0.05)
↓Weight of visceral adipose tissue in 0.5% group (p<0.05) |
|
Park, 201139
|
in vivo |
FCMPE, CMPE 0.1% and 0.5% of total diet, for 10 weeks |
HFD Sprague Dawley rat |
1) Normal diet (n=8)
2) HFD (n=8)
3) HFD+CMPE 0.1% (n=8)
4) HFD+CMPE 0.5% (n=8)
5) HFD+FCMPE 0.1% (n=8)
6) HFD+FCMPE 0.5% (n=8) |
none |
↓Body weight and FER in FCMPE 0.1%, 0.5% and
CMPE 0.1% group (p<0.05)
↓Weight of visceral adipose tissue in FCMPE 0.5% group (p<0.05) |
|
in vitro |
FCMPE,
CMPE and
FCB 25 or
50 μg/mL |
3T3-L1 cell |
1) Adipocyte
2) FCMPE 25 μg/mL
3) FCMPE 50 μg/mL
4) CMPE 25 μg/mL
5) CMPE 50 μg/mL
6) FCB 25 μg/mL
b7) FCB 50 μg/mL |
none |
↓Adipogenesis in all groups |
FCMPE,
CMPE and
FCB 25 mg/mL |
1) Preadipocyte
2) Adipocyte
3) FCMPE 25 μg/mL
4) CMPE 25 μg/mL
5) FCB 25 μg/mL |
↓TG content in all groups
↓GPDH activity in all groups |
|
Iwata, 201231
|
in vivo |
1% albedo TDF extracted by method of Prosky |
Wistar-Hannover GALAS rat |
1) Control (n=6)
2) 1% albedo TDF (n=6) |
none |
↓Weight of the cecum content (p<0.05)
↓Inhibition of pancreatic lipase activity (p<0.05)
↓Lipid content of feces (p<0.05) |
|
Park, 201335
|
in vivo |
CMPE 2 g per 100 g diet for 6 weeks |
C57BL/KsJ-db/db mice |
1) Control (n=10)
2) CMPE 2 g/100 g diet (n=10)
3) Rosiglitazone 0.001 g/100 g diet (n=10) |
rosiglitazone (0.001g/100 g diet) |
↓Body weight gain (p<0.05)
↓FER (p<0.05)
↓Total WAT weight (p<0.05) and epididymal WAT
↑plasma adiponectin level |
|
Jung, 201332
|
in vivo |
CMPE 100,
300 mg/kg, twice daily for 8 weeks. |
HFD Sprague
Dawley rat |
1) ND (n=10)
2) HFD (n=10)
3) HFD+CMPE 100 mg/kg (n=10)
4) HFD+CMPE 300 mg/kg (n=10) |
none |
↓Body weight in 300 mg/kg
group (p<0.001) |
|
Cho, 201430
|
in vivo |
CMP powder,
5%, 10%, 15%, for 4 weeks |
HFD Sprague Dawley rat |
1) ND
2) HFD
3) HFD+CMP 5%
4) HFD+CMP 10%
5) HFD+CMP 15% |
none |
↓Body weight in 15% group (p<0.05)
↓FER in 10% and 15% group (p<0.05)
↓Liver, kidney, testis weight in 10% and 15% group (p<0.05) |
|
Choi, 201423
|
in vitro |
CMPW 100 μg/ml |
OP9 cell |
1) Normal (pre-adipocyte)
2) Control (adipocyte)
3) CMPW 100 μg/ml
4) CMPIW 100 μg/ml |
none |
↓Lipid accumulation (p<0.01)
↓Protein expression of PPARγ2
↓Protein expression of Adiponectin |
|
Lim, 201527
|
in vitro |
CMPC, CMPE, 0.5 mg/mL each |
3T3-L1 cell |
1) Preadipocyte
2) Adipocyte
3) Sinetrol 0-0.5 mg/mL
4) CMPE 0.5 mg/mL
5) CMPC 0.5 mg/mL |
(0-0.5 mg/mL) of Sinetrol |
<CMPC>
↓Adipocyte differentiation (p<0.05)
↓mRNA levels of C/EBPα, PPARγ, SREBP1 (p<0.05)
↓Protein expression of C/EBPαand PPARγ(p<0.05)
↑Glycerol secretion (p<0.05)
<CMPE>
↓mRNA levels of PPARγ(p<0.05)
↑Glycerol secretion (p<0.05) |
|
Park, 201728
|
in vivo |
CMPE 1%, FCMPE 0.3% or FCMPE 1% for 13 weeks (ad libitum) |
HFD
C57BL/6J mice |
1) LFD (n=8)
2) HFD (n=8)
3) HFD+CMPE 1% (n=8)
4) HFD+FCMPE 0.3% (n=8)
5) HFD+FCMPE 1% (n=8) |
none |
↓Body weight, Epididymal fat weight and Adipocyte size of epididymal fat in FCMPE 0.3% and 1% group (p<0.05)
↓FER in all groups (p<0.05) |
|
Kang, 201840
|
clinical research |
CMP juice extract 18 mg for 4 weeks |
118 adult patients. BMI>23 kg/m2 |
1) 118 patients with BMI>23
(Female : 88, Male : 30) |
none |
↓Weight, BMI (p<0.0001)
↓Waist circumference (p=0.0002) |
|
Zeng, 202037
|
in vivo |
CBPW 1.04 g/kg for 4 weeks |
HFD Sprague-Dawley rat |
1) ND (n=8)
2) HFD (n=8)
3) HFD+1.04 mg/kg simvastatin (n=8)
4) HFD+CBPW 1.04 g/kg (n=8) |
1.04 mg/kg simvastatin |
↓Weight (p<0.05) |
|
Ke,202036
|
in vivo |
CBPE 0.2% and 0.5%, for 10 weeks |
HFD
C57BL/6 mice |
1) ND (n=8)
2) HFD (n=8)
3) HFD+0.1% resveratrol (n=8)
4) HFD+0.2% CBPE (n=8)
5) HFD+0.5% CBPE (n=8) |
0.1% resveratrol |
↓Body weight in both groups (p<0.05) |
a) Reduction of food efficiency ratio (FER)
(a.1) In vivo
CMPE 0.5% (241.9 mg/kg) applied to HFD rats 34 and CMPE 2 g per 100 g diet to diabetic mice 35 induced significant decrease of FER (p<0.05). Especially in CMPE 2 g group on the other hand, FER was significantly increased in RGZ group by 2.9-fold, suggesting greater weight gain efficiency. CMP powder 10% and 15% also reduced FER (p<0.05) 30. Aureobasidium pullulans fermented CMPE and CMPE 0.1%, 0.5% each were administered to HFD rats, and FER was significantly reduced (p<0.05), except for CMPE 0.5% group 39. As well, FCMPE processed by Aspergillus niger, which was found to contain naringenin, was administered to HFD mice 28. FCMPE 0.3%, 1% and CMPE 1% all lowered FER in HFD mice (p<0.05). b) Reduction of body weight, body mass index and waist circumference
(b.1) In vivo
Significant decrease in body weight gain was observed in CMPE 2 g per 100 g diet fed diabetic mice (p<0.05), whereas those in rosiglitazone (RGZ) group were markedly increased compared to both the control and CMPE groups 35. As well, body weight was significantly reduced by CMPE 300 mg/kg twice daily administration (p<0.001) 32. Daily feeding of CMP powder 15% had significant bodyweight reducing effect in HFD rats (p<0.05) 30. Weight lowering effect of fermented Chenpi at HFD rats also have been proved. Aureobasidium pullulans fermented CMPE and CMPE 0.1%, 0.5% each were fed, and body weight of FCMPE 0.1%, 0.5% and CMPE 0.1% were significantly reduced (p<0.05) 39. Especially FCMPE 0.5% group, which was the most effective, lost more than 40 g compared to control group. Aspergillus niger fermented CMPE 0.3%, 1% and CMPE 1% were applied, and both doses of FCMPE significantly reduced body weight (p<0.05) 28. A clinical research about CMP also has been conducted. CMP juice extract administration to 118 adult patients of BMI over 23 kg/m 2 resulted significant loss of body weight (p<0.0001) 40. Several in vivo studies about CBP are also presented. CBPW 1.04 g/kg (p<0.05) 36, CBPE 0.2% and 0.5% (p<0.05) 37 led to significant body weight reduction in HFD rats. (b.2) Clinical research
A clinical research, 4 weeks application of CMP juice extract to 118 adult patients of BMI over 23 kg/m 2 resulted in reduction of BMI (p<0.0001) and waist circumference (p=0.0002) 40. c) Reduction of visceral adipose tissue
(c.1) In vivo
CMPE 0.5% 34 and Aureobasidium pullulans fermented CMPE 0.5% 39 significantly reduced weight of visceral adipose tissue (p<0.05). In addition, Aspergillus niger fermented CMPE 0.3% and 1% lowered epididymal fat weight and adipocyte size of epididymal fat (p<0.05) in HFD rats 28. In diabetic mice, CMPE 2 g per 100 g diet significantly reduced total white adipose tissue (WAT) weight (p<0.05) and epididymal WAT weight 35. Also in this case, total WAT weight of RGZ group was markedly increased compared to both the control and CMPE group. CMP powder 10% and 15% lowered liver, kidney and testis weight of HFD fed rats (p<0.05) 30. d) Suppression of TG biosynthesis
(d.1) In vitro
Glycerol 3-phosphate dehydrogenase (GPDH) uses NAD as a coenzyme, and transfers dihydroxyacetone phosphate into glycerol-3-phosphate 63,64. It is usually activated in case of differentiation of preadipocyte into adipocyte. GPDH activity seems to be elevated in adipose tissue of obese subjects 65. CMPE, Aureobasidium pullulans fermented CMPE and fermented citrus peel culture broth powder (FCB) 25 mg/mL suppressed GPDH activity in 3T3-L1 cell 39. e) Suppression of lipid accumulation
(e.1) In vitro
CMPE, Aureobasidium pullulans fermented CMPE and FCB 25 mg/mL lowered TG content in 3T3-L1 cell 39. In 2014 study, CMPW and Citrus Unshiu Pericarpium Immaturus water extract (CMPIW), each 100 μg/ml, were administered to OP9 cell 23. As a result, both significantly inhibited lipid accumulation and CMPIW was found out to be more effective than CMPW (p<0.01). f) Stimulation of lipid excretion through feces
(f.1) In vivo
Fibrous element of Chenpi also has shown anti-obesitic effect. 1% albedo total dietary fiber (TDF), extracted by method of Prosky, was fed to Wistar-Hannover GALAS rats. As a result, weight of the cecum content and lipid content of feces were significantly reduced (p<0.05) 31. g) Suppressing adipogenesis
(g.1) In vitro
In 3T3-L1 cells, CMPE, Aureobasidium pullulans fermented CMPE and FCB 25, 50 mg/mL suppressed adipogenesis 39. h) Regulation of lipid metabolism
(h.1) In vitro
Adiponectin, which promotes insulin activity, is suppressed by inflammatory cytokines such as IL-6, tumor necrosis factor α (TNFα) and IFN-γ 66. Decrease of adiponectin level was observed in hepatic steatosis and T2DM model (OP9 cell) 67,68. PPARγ, a transcription factor which is strongly expressed in adipose tissue, is involved in adipogenesis differentiation, carbohydrate, and lipid metabolism 69. CMPW and CMPIW down regulated the protein expression of peroxisome-proliferator activated receptor γ2 (PPARγ2) and Adiponectin 23. In addition, CMPI was found to have better lipid-lowering and PPARγ2 suppressing effect than CMP. CMPW 100 μg/ml suppressed protein expression of PPARγ2 in OP9 cell 23. Cytolase is a compound of glycosidases removed from Aspergillus niger, which bioconverted CMP into aglycoside forms. In 3T3-L1 cells, Citrus unshiu with cytolase (CMPC) 0.5 mg/mL significantly reduced mRNA levels of CCAAT/Enhancer-binding Protein α (C/EBPα), SREBP1, protein expression of C/EBPα and PPARγ (p<0.05) 27. It also significantly suppressed adipocyte differentiation (p<0.05), showing better effect than that of Sinetrol positive control group. In all CMPC, CMPW and CMPE 0.5 mg/mL groups, mRNA level of PPARγ was reduced, and glycerol secretion, which is involved in lipolysis, was increased (p<0.05). (h.2) In vivo
Anti-obesitic activity related to lipase activity of Chenpi dietary fiber was evaluated. 1% albedo TDF extracted by method of Prosky significantly suppressed inhibition of pancreatic lipase activity in Wistar-Hannover GALAS rat (p<0.05) 31. Also, CMPE 2 g per 100 g diet fed to diabetic mice for 6 weeks increased plasma adiponectin level 35.
4. Hepatic steatosis
IR has strong correlation to hepatic steatosis, a previous phase of NAFLD 70. Resistance to insulin activity on hepatic gluconeogenesis leads to an excessive lipid accumulation in the liver 71. Susceptibility of IR and type 2 DM in patients with NAFLD has been studied 72. The effect of Chenpi on hepatic steatosis is analyzed below ( Table 4).
Table 4
Summary of studies on Chenpi in Hepatosteatosis
|
Reference and year |
Method |
Dose and duration |
Experimental model |
Control and intervention groups |
Positive control |
Effect |
|
Park, 201335
|
in vivo |
CMPE 2 g per 100 g diet for 6 weeks |
C57BL/
KsJ-db/db mice |
1) Control (n=10)
2) CMPE 2 g/100 g diet (n=10)
3) Rosiglitazone 0.001 g/100 g diet (n=10) |
rosiglitazone (0.001g/ 100 g diet) |
↓Hepatic TG, TC, FFA level and liver weight (p<0.05)
↓Lipid droplet accumulation in liver and liver size
↓Liver weight (p<0.05)
↓Hepatic FAS, ME activity (p<0.05)
↓Activity of PAP (p<0.05)
↓mRNA level of HMGR (p<0.05)
↑Hepatic CMPT mRNA expression,
fatty acid β-oxidation (p<0.05) |
|
Cho, 201430
|
in vivo |
5%, 10% or 15% CMP powder for 4 weeks |
HFD Sprague Dawley rat |
1) ND
2) HFD
3) HFD+CMP 5%
4) HFD+CMP 10%
5) HFD+CMP 15% |
none |
↓Hepatic total lipid level in 10% and 15% group (p<0.05)
↓Hepatic TC level in 10,
15% group (p<0.05)
↓Hepatic TG level in 10% group (p<0.05)
↓Hepatic lipid level accumulation |
|
Lim, 201433
|
in vivo |
CMPE 30,
100 or 300 mg/kg for 8 weeks |
OVX-Sprague Dawley rat |
1) Sham (n=12)
2) OVX (n=12)
3) OVX+E2 10 μg/kg (n=12)
4) OVX+CMPE 30 mg/kg (n=12)
5) OVX+CMPE 100 mg/kg (n=12)
6) OVX+CMPE 300 mg/kg (n=12) |
E2 10 μg/kg |
↓Serum GOT, GPT level (p<0.05)
↓Hepatic TC, TG level in 100, 300 mg/kg group (p<0.05)
↓Hepatic fatty deposition in hepatocytes in 300 mg/kg group |
|
Park, 201728
|
in vivo |
CMPE 1%, FCMPE 0.3% or FCMPE 1% for 13 weeks (ad libitum) |
HFD C57BL/6J mice |
1) LFD (n=8)
2) HFD (n=8)
3) HFD+CMPE 1% (n=8)
4) HFD+FCMPE 0.3% (n=8)
5) HFD+FCMPE 1% (n=8) |
none |
↓Hepatic lipid accumulation in FCMPE 0.3% and 1% group (p<0.05) |
|
Kwak, 202038
|
in vivo |
CMPW 200 or 300 mg/kg for 4 weeks |
diabetic C57BL/6 mice induced with STZ |
1) Diabetic mice (n=6)
2) Diabetic mice+CMPW 200 mg/kg (n=6)
3) Diabetic mice+CMPW 300 mg/kg (n=6) |
none |
↓Liver and kidney weight (p<0.05) and size. |
|
Ke, 202036
|
in vivo |
CBPE 0.2% and 0.5%, for 10 weeks |
HFD
C57BL/6J mice |
1) ND (n=8)
2) HFD (n=8)
3) HFD+0.1% resveratrol (n=8)
4) HFD+0.2% CBPE (n=8)
5) HFD+0.5% CBPE (n=8) |
0.1% resveratrol |
↓Lobule structure, micro steatosis and excessive lipid droplet accumulation in the liver, in both groups.
↓NAS in 0.5% group (p<0.05)
↓Hepatic TG level in both groups (p<0.05)
↓Hepatic TC level in 0.5% group (p<0.05)
↓Serum MDA level in both groups (p<0.05)
↓Hepatic MDA level and hepatic LPO in 0.5% group (p<0.05)
↑Hepatic GR level in both groups (p<0.05)
↑mRNA expressions of Nrf2 in both groups (p<0.05)
↑mRNA expressions of Prdx and NQ-O1 in 0.5% group (p<0.05) |
a) Reduction of liver weight and size
(a.1) In vivo
CMPE 2 g per 100 g diet fed to diabetic mice significantly reduced liver weight (p<0.05, p<0.01 each) and size 35. CMPW 200 and 300 mg/kg significantly decreased liver and kidney weight (p<0.05) and size in STZ induced diabetic mice 38. b) Suppression of hepatic lipid accumulation
(b.1) In vivo
Various studies have shown reduction of hepatic lipid accumulation in liver. It was shown at CMPE 300 mg/kg in OVX rats 33. Particularly, CMPE 2 g per 100 g diet administration to diabetic mice lowered hepatic lipid levels were in comparison to those in RGZ 0.001 g per 100 g diet group, which were conversely increased 35. Similar results were shown at CMP powder 5%, 10%, 15% 30 and Aspergillus niger fermented CMPE 0.3%, CMPE 1% (p<0.05) 28 in HFD mice. CBPE 0.2%, 0.5% in HFD mice lowered lobule structure, micro steatosis and excessive lipid droplet accumulation in the liver, in both groups 37. Also, 10% and 15% CMP powder in HFD rats resulted in reduction of hepatic total lipid level (p<0.05) 30. c) Reduction of hepatic lipid levels
(c.1) In vivo
CMPE 2 g per 100 g diet lowered hepatic TG, TC and FFA level in diabetic mice (p<0.05) 35. Freeze dried Chenpi also had lipid lowering effect. Hepatic TC level was reduced by 10%, 15% freeze dried CMP medication, and hepatic TG level was reduced by 10% CMP powder medication 30. In OVX rats, CMPE 100 and 300 mg/kg administration induced decreased of hepatic TC and TG level (p<0.05) 33. In HFD mice CBPE 0.2%, 0.5% all reduced hepatic TG level, significantly (p<0.05) 37. In addition, CBPE 0.5% apply lowered hepatic TC level (p<0.05) 37. d) Suppression of hepatic lipid synthesizing enzyme activity
(d.1) In vivo
CMPE 2 g per 100 g diet fed to diabetic mice resulted in down regulation of hepatic lipid regulating enzyme activities and mRNA expression 35. Hepatic FAS, malic enzyme (ME) activities, which implies fatty acid synthesis, were significantly down-regulated, even compared to that of RGZ group (p<0.05). Activity of phosphatidate phosphohydrolase (PAP), related to TG synthesis, was down-regulated (p<0.05), and reverse effect was shown in RGZ group. The rate-limiting enzyme in cholesterol synthesis, m-RNA level of HMGR, was significantly lowered (p<0.05). Also, hepatic CMPT mRNA expression and fatty acid β-oxidation were significantly up-regulated (p<0.05). Administration of 0.2% and 0.5% CBPE to HFD mice proved anti-hepatic oxidative stress effect 36. In both groups, serum malondialdehyde (MDA) level was lowered (p<0.05) and hepatic glutathione reductase (GR) level was elevated (p<0.05). And in 0.5% group, hepatic MDA, and hepatic lipid peroxidation (LPO) level were reduced (p<0.05). Lastly, it upregulated hepatic mRNA expression of nuclear factor - like 2 (Nfr2) signaling genes and ameliorated inflammatory cytokines. Up-regulations of nuclear factor erythroid 2-related factor 2 (Nrf2) mRNA expressions in both groups (p<0.05) and peroxiredoxin (Prdx) and Nicotinamide Adenine Dinucleotide Phosphate Hydrogen quinone oxidoreductase (NQ-O1) mRNA expressions in 0.5% group (p<0.05) were observed. e) Reduction of non-alcoholic fatty liver activity score
(e.1) In vivo
0.5% CBPE fed to HFD mice confirmed significantly lowered NAFLD activity score (NAS) (p<0.05).
5. Inflammation
Reports have proved the relation of inflammationin the pathogenesis of MetS associated disorder, T2DM 73-76. In addition, it is known that chronic inflammation is closely related to T2DM and MetS 77. The effect of Chenpi on inflammation is analyzed below ( Table 5).
Table 5
Summary of studies on Chenpi in Inflammation
|
Reference and year |
Method |
Dose and duration |
Experimental model |
Control and intervention groups |
Positive control |
Effect |
|
Park, 201335
|
in vivo |
CMPE
2 g/100 g diet for 6 weeks |
57BL/KsJ-db/db mice |
1) Control (n=10)
2) CMPE 2 g/100 g diet (n=10)
3) Rosiglitazone 0.001 g/100 g diet (n=10) |
rosiglitazone (0.001 g/100 g diet) |
↓Serum IL-6, TNF-α,
IFN-γ level (p<0.05)
↓Serum MCP-1 level
↓Hepatic MCP-1 mRNA expression
↑Adiponectin and IL-10 level |
|
Ke, 202036
|
in vivo |
CBPE 0.2% and 0.5% for 10 weeks |
HFD
C57BL/6 mice |
1) ND (n=8)
2) HFD (n=8)
3) HFD+0.1% resveratrol (n=8)
4) HFD+0.2% CBPE (n=8)
5) HFD+0.5% CBPE (n=8) |
0.1% resveratrol |
↓TNF-α, IL-6 and IL-1β mRNA levels in both groups (p<0.05) |
a) Reduction of tumor necrosis factor and interferon level
(a.1) In vivo
CMPE 2 g per 100 g diet was fed to diabetic mice, for 6 weeks, and RGZ was selected as positive control 35. In conclusion, reduction of inflammatory biomarkers in blood and liver were observed, which indicate that CMPE attenuated diabetes-induced inflammatory responses. Plasma interleukin (IL-6), tumor necrosis factor-α (TNF-α) and IFN-γ level were significantly decreased (p<0.05). In addition, adiponectin (plasma anti-inflammatory) and IL-10 level were increased but RGZ group lowered them. Elevation of plasma proinflammatory cytokines such as interleukin-6 (IL-6), TNFα and interferon (IFN)-γ 78-81, and a decrease of IL-10 is associated to T2DM 82,83. 0.2% and 0.5% of CPBE fed to HFD mice confirmed inhibition of hepatic inflammatory factors, as well 36. TNF-α, IL-6 and IL-1β mRNA levels were lowered in both groups (p<0.05). Especially, suppression of IL-1β activity was significantly better in CBPE groups, compared to positive control group (0.1% resveratrol). b) Reduction of human monocyte chemoattractant protein-1 level
(b.1) In vivo
Especially, an inflammatory chemokine human monocyte chemoattractant protein-1 is linked to insulin resistance, leading to NAFLD 78. CMPE 2 g per 100 g applied to HFD mice reduced plasma MCP-1 level and hepatic MCP-1 mRNA expression. However, RGZ group did not alter this pro-inflammatory marker in plasma and liver.
IV. Conclusion and Discussion
Recently, various in vitro, in vivo, and clinical studies have substantiated that Chenpi has beneficial effects against T2DM, HL, obesity, hepatic steatosis, and inflammation, resulting in amelioration of MetS. According to previous discussion in this article, Chenpi has effect on T2DM through glucose lowering, insulin secretion stimulation, glucose regulation, gluconeogenesis suppression, beta cell protection and anti-inflammatory activity. It exerts effect on HL through plasma lipid regulation, apolipoprotein reduction, plasma lipid metabolism regulation and preventing atherosclerosis. In aspects of obesity, Chenpi exerts decrease of FER, body weight, BMI, visceral adipose tissue, TG bio synthesis, lipid accumulation and adipogenesis. Lipid metabolism regulation and lipid excretion through feces are also included. Chenpi has also been substantiated hepatic steatosis ameliorating effect, by reduction of liver weight and size, hepatic lipid accumulation, hepatic lipid level, NAFLD activity score and suppression of hepatic lipid synthesizing activity.
Chenpi has effect of regulating qi (理氣), dissolving abscesses (散結), drying dampness (燥濕), relieving hiccup (止呃), suppressing cough (止咳), ameliorating diarreha (止瀉), resolving phlegm (化痰), removing food stagnation (導滯), eliminating phlegm (消痰), increaseing appetite (開胃), relieving strangury (通淋). It has been used in various symptoms, comprising vomiting, hiccup, lost of appetite, cough, sputum and dyspepsia 84. It is included in various decotions for digestive symptoms and invigorants. Gamibojungikgi-tang 85, gamiyukgunja-tang 86, yukgunja -tang 87, gamiyijin-tang 88, gamigwakhyangjeonggi-san 89 had been reported for their anti-obesitic effets. Also, bojungikgi-tang 90 for its hypolipidemic effect, sopyung-tang 91, gamiyukmijihwang-tang 92 for their hypoglycemic effect, and saenggangeonbi-tang 93,94 for its anti-fatty liver effect had been reported. All mentioned herbal decotions contains Chenpi as common herbal medicine. It implies possibility of Chenpi as treatment of various metabolic diseases. However, lack of clinical or reviewing researches so far built limits to prove the efficacy of Chenpi. Therefore, our research targeted the potentials of Chenpi for MetS treatment. Chenpi appears to be potential treatment of chronic metabolic disorders, as hypoglycemic, hypolipidemic, anti-obesitic, fatty liver-ameliorating and anti-inflammatory effects were broadly studied. On the basis of our research, we look forward to continuous studies and to prove applicability of Chenpi as Mets treatment, in clinical use. There were some limitations on discussing effects of Chenpi on our research. First, every dose of Chenpi used in experiments were not quantified, so it was not enough to compare exact effect with same amount of experimental material. Second, there were some studies about effect of bioconversed Chenpis, claiming that anti-diabetic, anti-hyperlipidemic and anti-obesitic effect would be reinforced by bioconversion. Though, bioconversed flavonoids were not standardized due to all different mycotoxins used in fermentation, resulting in inexact comparison. Finally, more clinical studies about efficacy of Chenpi in above-mentioned conditions (diabetes, hyperlipidemia, obesity, hepatic steatosis and inflammation) should be required to provide evidence of clinical use.
References
1. Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep 2018:20(2):12doi:10.1007/s11906-018-0812-z.    2. Elaine W, Wat E, Wang Y, Law HW, Koon CM, Lau KM, et al. An in vitro and in vivo study of a 4-herb formula on the management of diet-induced metabolic syndrome. Phytomedicine 2018:42:112–25.   3. Lim S, Shin H, Song JH, Kwak SH, Kang SM, Yoon JW, et al. Increasing prevalence of metabolic syndrome in Korea:the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes care 2011:34(6):1323–28. doi: 10.2337/dc10-2109 [published Online First: 04/19].    4. National Health and Nutrition Survey. Prevalance of Metabolic Syndrome 2012:
5. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014:2014:943162–62. doi:10.1155/2014/943162 [published Online First:03/11].    6. Huang PL. A comprehensive definition for metabolic syndrome. Disease models &mechanisms 2009:2(5-6):231–7. doi:10.1242/dmm.001180 [published Online First:2009/05/02].  7. Grundy SM. Metabolic Syndrome:A Multiplex Cardiovascular Risk Factor. The Journal of Clinical Endocrinology &Metabolism 2007:92(2):399–404. doi:10.1210/jc.2006-0513.
8. Cooney MT, Dudina A, De Bacquer D, Wilhelmsen L, Sans S, Menotti A, et al. HDL cholesterol protects against cardiovascular disease in both genders, at all ages and at all levels of risk. Atherosclerosis 2009:206(2):611–6. doi:10.1016/j.atherosclerosis.2009.02.041.   9. Karr S. Epidemiology and management of hyperlipidemia. Am J Manag Care 2017:23(9 Suppl):S139–48.  10. Shah RV, Goldfine AB. Statins and risk of new-onset diabetes mellitus. Circulation 2012:126(18):e282–4. doi:10.1161/CIRCULATIONAHA.112.122135.   11. Haas AV, McDonnell ME. Pathogenesis of Cardiovascular Disease in Diabetes. Endocrinol Metab Clin North Am 2018:47(1):51–63.   12. Wang GS, Hoyte C. Review of Biguanide (Metformin) Toxicity. Journal of intensive care medicine 2019:34(11-12):863–76. doi:10.1177/0885066618793385 [published Online First:2018/08/22].   13. Babajafari S, Nikaein F, Mazloomi SM, Zibaeenejad MJ, Zargaran A. A review of the benefits of Satureja species on metabolic syndrome and their possible mechanisms of action. Journal of evidence-based complementary &alternative medicine 2015:20(3):212–23. doi:10.1177/21565∑4564188 [published Online First:2015/01/08].  14. Vahabzadeh M, Amiri N, Karimi G. Effects of silymarin on metabolic syndrome:a review. Journal of the science of food and agriculture 2018:98(13):4816–23. doi:10.1002/jsfa.9115 [published Online First:2018/05/08].  15. Razavi BM, Hosseinzadeh H. Saffron:a promising natural medicine in the treatment of metabolic syndrome. Journal of the science of food and agriculture 2017:97(6):1679–85. doi:10.1002/jsfa.8134 [published Online First:2016/11/20].  16. Wang J, Ke W, Bao R, Hu X, Chen F. Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome:a review. Annals of the New York Academy of Sciences 2017:1398(1):83–98. doi:10.1111/nyas.13375 [published Online First:2017/05/16].   17. Lyu JH, Lee HT. Effects of dried Citrus unshiu peels on gastrointestinal motility in rodents. Archives of pharmacal research 2013:36(5):641–8. doi:10.1007/s12272-013-0080-z [published Online First:2013/03/07].   18. Lu Y, Zhang C, Bucheli P, Wei D. Citrus flavonoids in fruit and traditional Chinese medicinal food ingredients in China. Plant foods for human nutrition (Dordrecht, Netherlands) 2006:61(2):57–65. doi:10.1007/s11130-006-0014-8 [published Online First:2006/07/04].   19. Tejada S, Pinya S, Martorell M, Capó X, Tur JA, Pons A, et al. Potentials Anti-inflammatory Effects of Hesperidin from the Genus Citrus. Current medicinal chemistry 2018:25(37):4929–45. doi:10.2174/0929867324666170718104412 [published Online First:2017/07/20].  20. Hajialyani M, Hosein Farzaei M, Echeverría J, Nabavi SM, Uriarte E, Sobarzo-Sánchez E. Hesperidin as a Neuroprotective Agent:A Review of Animal and Clinical Evidence. Molecules (Basel, Switzerland) 2019:24(3):648doi:10.3390/molecules24030648 [published Online First:2019/02/15].   21. Aggarwal V, Tuli HS, Thakral F, Singhal P, Aggarwal D, Srivastava S, et al. Molecular mechanisms of action of hesperidin in cancer:Recent trends and advancements. Experimental biology and medicine (Maywood, NJ) 2020:245(5):486–97. doi:10.1177/1535370220903671 [published Online First:2020/02/14].
22. Mas-Capdevila A, Teichenne J, Domenech-Coca C, Caimari A, Del Bas JM, Escoté X, et al. Effect of Hesperidin on Cardiovascular Disease Risk Factors:The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients 2020:12(5):1488doi:10.3390/nu12051488 [published Online First:2020/05/24].   23. Choi ES. Effect of Citri Unshiu Pericarpium and Citri Unshiu Pericarpium Immaturus on Differentiation of Pre-adipocyte. Department of Korean Pharmacy Graduate School of Wonkwang University 2014:
24. Jo HK. Anti-obesity Activities of Ethanol Extract of Citrus Peel in 3T3-L1 Preadipocytes. Department of Korean Medicine Graduate School, Dong-Eui University 2016:
25. Kim JN, Lim EY, Kim YT, Kim HW, Kim BJ. Effects of the herbal medicines on voltage-dependent K+2 channels. Pharmacognosy Magazine 2019:15(63):369–77.
26. Kim SH, Park HJ, Kim KJ, Kim MJ, Lee JA, Lee AR, et al. Antioxidant Activity of Citrus Peel and Effect on its Glucose Metabolism in L6 Rat Skeletal Muscle Cell. Kor J Herbology 2018:33(4):101–9.
27. Lim HJ, Yeo EJ, Song EJ, Chang YH, Han BK, Choi HJ, et al. Bioconversion of Citrus unshiu peel extracts with cytolase suppresses adipogenic activity in 3T3-L1 cells. Nutrition research and practice 2015:9(6):599–605. doi:10.4162/nrp.2015.9.6.599 [published Online First:2015/12/04].    28. Park HS. Effect of citrus unshiu peel extract fermented by Aspergillus niger on obesity and hyperglycemia in highfat diet fed C57BL/6J mice. 서울대학교 대학원 2017:
29. Youn Y, Kim YS. Inhibitory effects of Citrus unshiu pericarpium extracts on palmitate-induced lipotoxicity in HepG2 cells. Food science and biotechnology 2016:25(6):1709–17. doi:10.1007/s10068-016-0262-9 [published Online First:2016/12/31].    30. Cho HE. Quality Characteristics of Ginpimook and Effect of Citri Pericarpium on Lipid Metabolism in Rats Fed a High-Fat Diet. Department of Diets &Health Care, Graduate School of Industry, Myongji University 2014:
31. Iwata E, Hotta H, Goto M. Hypolipidemic and bifidogenic in the dietary fiber prepared from Mikan (Japanese mandarin orange:Citrus unshiu) albedo. Journal of nutritional science and vitaminology 2012:58(3):175–80. doi:10.3177/jnsv.58.175 [published Online First:2012/08/11].  32. Jung S. Anti-obesity effect of Citrus unshiu peel in rats fed a high-fat diet. Department of Science in Korean Medicine, Graduate School, Kyung Hee University 2013:
33. Lim DW, Lee Y, Kim YT. Preventive effects of Citrus unshiu peel extracts on bone and lipid metabolism in OVX rats. Molecules (Basel, Switzerland) 2014:19(1):783–94. doi:10.3390/molecules19010783 [published Online First:2014/01/15].    34. Park CH, Jung HK, Jeong YS, Hong JH, Lee GD, Park CD. Effects of Citrus Peel Ethanol Extract on the Serum Lipid and Body Fat of High-fat-diet-fed Rats. The Korean Society of Food Preservation 2011:18(4):567–74.  35. Park HJ, Jung UJ, Cho SJ, Jung HK, Shim S, Choi MS. Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose- and lipid- regulating enzymes in db/db mice. The Journal of nutritional biochemistry 2013:24(2):419–27. doi:10.1016/j.jnutbio.2011.12.009 [published Online First:2012/06/15].   36. Ke Z, Zhao Y, Tan S, Chen H, Li Y, Zhou Z, et al. Citrus reticulata Blanco peel extract ameliorates hepatic steatosis, oxidative stress and inflammation in HF and MCD diet-induced NASH C57BL/6 J mice. The Journal of nutritional biochemistry 2020:83:108426doi:10.1016/j.jnutbio.2020.108426 [published Online First:2020/06/20].   37. Zeng W, Huang KE, Luo Y, Li DX, Chen W, Yu XQ, et al. Nontargeted urine metabolomics analysis of the protective and therapeutic effects of Citri Reticulatae Chachiensis Pericarpium on high-fat feed-induced hyperlipidemia in rats. Biomedical chromatography :BMC 2020:34(4):e4795doi:10.1002/bmc.4795 [published Online First:2020/01/23].   38. Kwak KH. Antidiabetic Effect of Pericarpium Citri Reticulatae Extract. Professional Graduate School of Korean Medicine, Wonkwang University 2020:
39. Park CD. A study on the bioconversion of citrus peel flavonoid and antiobesity effect using Aureobasidium pullulan. Department of Biology, Graduate School, Keimyung University 2011:
40. Kang SI, Song SY, Lee JS, Chang, HK, Lee SH. Clinical Investigations of the Effect of Citrus unshiu Peel Pellet on Obesity and Lipid Profile. Evidence-based complementary and alternative medicine :eCAM 2018:2018:4341961doi:10.1155/2018/4341961 [published Online First:2018/10/18].    41. Lee JS, Do EJ, Kwak MA, Park HJ, Ha ID, Sung KJ, et al. Clinical Trial to Evaluate the Efficacy of Extract of Citri Pericarpium on Serum Lipid Profiles in Subjects:a Randomized, Double-blind. Kor J Herbology 2011:26(1):125–32.
42. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes research and clinical practice 2010:87(1):4–14. doi:10.1016/j.diabres.2009.10.007 [published Online First:2009/11/10].   43. Haidara M, Mikhailidis DP, Yassin HZ, Dobutovic B, Smiljanic KT, Soskic S, et al. Evaluation of the possible contribution of antioxidants administration in metabolic syndrome. Current pharmaceutical design 2011:17(33):3699–712. doi:10.2174/138161211798220882 [published Online First:2011/11/15].   44. Atwater I, Ribalet B, Rojas E. Mouse pancreatic beta-cells:tetraethylammonium blockage of the potassium permeability increase induced by depolarization. The Journal of physiology 1979:288(1):561–74.   45. Henquin J, Meissner H, Preissler M. 9-aminoacridine -and tetraethylammonium-induced reduction of the potassium permeability in pancreatic B-cells:Effects on insulin release and electrical properties. Biochimica et Biophysica Acta (BBA)-General Subjects 1979:587(4):579–92.
46. Henquin JC. Tetraethylammonium potentiation of insulin release and inhibition of rubidium efflux in pancreatic islets. Biochemical and biophysical research communications 1977:77(2):551–6.   47. MacDonald PE, Sewing S, Wang J, Joseph JW, Smukler SR, Sakellaropoulos G, et al. Inhibition of Kv2. 1 voltage-dependent K+channels in pancreatic β-cells enhances glucose-dependent insulin secretion. Journal of Biological Chemistry 2002:277(47):44938–45.  48. Kim SM, Lee YM, Kim MJ, Nam SY, Kim SH, Jang HH. Effects of Agrimonia pilosa Ledeb. Water Extract on α-Glucosidase Inhibition and Glucose Uptake in C2C12 Skeletal Muscle Cells. Korean J Food &Nutr 2013:26(4):806–13.  49. Jeon SY, Park JY, Kim SO, Lee ES, Koo JS, Kim MR. Water extract of fermented new korean medicinal mixture (F-MAPC) controls intracellula adipogenesis and Glut-4 dependent glucose uptake in 3T3-L1 adipocytes and L6 myoblasts. The Korea Journal of Herbology 2014:29(1):45–52.  50. Valera A, Pujol A, Pelegrin M, Bosch F. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proceedings of the National Academy of Sciences of the United States of America 1994:91(19):9151–4. doi:10.1073/pnas.91.19.9151 [published Online First:1994/09/13].    51. Tachibana S, Sato K, Cho Y, Chiba T, Schneider WJ, Akiba Y. Octanoate reduces very low-density lipoprotein secretion by decreasing the synthesis of apolipoprotein B in primary cultures of chicken hepatocytes. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2005:1737(1):36–43. doi:https://doi.org/10.1016/j.bbalip.2005.09.001.  52. Dobiásová M, Frohlich J. The new atherogenic plasma index reflects the triglyceride and HDL-cholesterol ratio, the lipoprotein particle size and the cholesterol esterification rate:changes during lipanor therapy. Vnitrni lekarstvi 2000:46(3):152–6. [published Online First:2000/10/26].  53. Fernández-Macías JC, Ochoa-Martínez AC, Varela-Silva JA, Pérez-Maldonado IN. Atherogenic Index of Plasma:Novel Predictive Biomarker for Cardiovascular Illnesses. Archives of medical research 2019:50(5):285–94. doi:10.1016/j.arcmed.2019.08.009 [published Online First:2019/10/09].   54. Shen S, Lu Y, Qi H, Li F, Shen Z, Wu L, et al. Association between ideal cardiovascular health and the atherogenic index of plasma. Medicine 2016:95(24):e3866doi:10.1097/md.0000000000003866 [published Online First:2016/06/17].    55. Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index:correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clinical biochemistry 2001:34(7):583–8. doi:10.1016/s0009-9120(01)00263-6 [published Online First:2001/12/12].  56. Takahashi MM, de Oliveira EP, de Carvalho AL, de Souza Dantas LA, Burini FH, Portero-McLellan KC. Metabolic syndrome and dietary components are associated with coronary artery disease risk score in free-living adults:a cross-sectional study. Diabetology &metabolic syndrome 2011:3:7doi:10.1186/1758-5996-3-7 [published Online First:2011/05/11].
57. Bok SH, Lee SH, Park YB, Bae KH, Son KH, Jeong TS, et al. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl -glutaryl-CoA reductase and acyl CoA:cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. The Journal of nutrition 1999:129(6):1182–5. doi:10.1093/jn/129.6.1182 [published Online First:1999/06/04].  58. Akpınar O, Bozkurt A, Acartürk E, Seydaoğlu G. A new index (CHOLINDEX) in detecting coronary artery disease risk. Anadolu kardiyoloji dergisi :AKD =the Anatolian journal of cardiology 2013:13(4):315–9. doi:10.5152/akd.2013.098 [published Online First:2013/03/28].  59. Oguejiofor OC, Onwukwe CH, Odenigbo CU. Dyslipidemia in Nigeria:prevalence and pattern. Annals of African medicine 2012:11(4):197–202. doi:10.4103/1596-3519.102846 [published Online First:2012/10/30].  60. Fang P, Shi M, Zhu Y, Bo P, Zhang Z. Type 2 diabetes mellitus as a disorder of galanin resistance. Experimental Gerontology 2016:73:72–7. doi:https://doi.org/10.1016/j.exger.2015.11.007.   61. Seidell JC, Halberstadt J. The Global Burden of Obesity and the Challenges of Prevention. Annals of Nutrition and Metabolism 2015:66(suppl 2):7–12. doi:10.1159/000375143.  62. Obesity:preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series 2000:894(i-xii):1–253. [published Online First:2001/03/10].
63. Koekemoer TC, Litthauer D, Oelofsen W. Isolation and characterization of adipose tissue glycerol -3-phosphate dehydrogenase. The international journal of biochemistry &cell biology 1995:27(6):625–32. doi:10.1016/1357-2725(95)00012-e [published Online First:1995/06/01].
64. Pairault J, Green H. A study of the adipose conversion of suspended 3T3 cells by using glycerophosphate dehydrogenase as differentiation marker. Proceedings of the National Academy of Sciences of the United States of America 1979:76(10):5138–42. doi:10.1073/pnas.76.10.5138 [published Online First:1979/10/01].    65. Swierczynski J, Zabrocka L, Goyke E, Raczynska S, Adamonis W, Sledzinski Z. Enhanced glycerol 3-phosphate dehydrogenase activity in adipose tissue of obese humans. Molecular and cellular biochemistry 2003:254(1-2):55–9. doi:10.1023/a:1027332523114 [published Online First:2003/ 12/17].  66. Matsuzawa Y. Therapy Insight:adipocytokines in metabolic syndrome and related cardiovascular disease. Nature clinical practice Cardiovascular medicine 2006:3(1):35–42. doi:10.1038/ncpcardio0380 [published Online First:2006/01/05].   67. Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH:TNF-alpha or adiponectin? Hepatology (Baltimore, Md) 2004:40(1):46–54. doi:10.1002/hep.20280 [published Online First:2004/07/09].  68. Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. The Journal of clinical investigation 2003:112(1):91–100. doi:10.1172/jci17797 [published Online First:2003/07/04].    69. Festuccia WT, Blanchard PG, Turcotte V, Laplante M, Sariahmetoglu M, Brindley DN, et al. The PPARgamma agonist rosiglitazone enhances rat brown adipose tissue lipogenesis from glucose without altering glucose uptake. American journal of physiology Regulatory, integrative and comparative physiology 2009:2 96(5):R1327–35. doi:10.1152/ajpregu.91012.2008 [published Online First:2009/02/13].
70. Yki-Järvinen H, Westerbacka J. The fatty liver and insulin resistance. Current molecular medicine 2005:5(3):287–95. doi:10.2174/1566524053766031 [published Online First:2005/05/17].   71. Brown MS, Goldstein JL. Selective versus total insulin resistance:a pathogenic paradox. Cell metabolism 2008:7(2):95–6. doi:10.1016/j.cmet.2007.12.009 [published Online First:2008/02/06].   72. Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels:a role for insulin resistance and diabetes. Hepatology (Baltimore, Md) 2008:48(3):792–8. doi:10.1002/hep.22429 [published Online First:2008/08/30].  73. Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes &metabolic syndrome 2019:13(2):1165–72. doi:10.1016/j.dsx.2019.01.040 [published Online First:2019/07/25].  74. Karstoft K, Pedersen BK. Exercise and type 2 diabetes:focus on metabolism and inflammation. Immunology and cell biology 2016:94(2):146–50. doi:10.1038/icb.2015.101 [published Online First:2015/11/17].  75. Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Current diabetes reports 2013:13(3):435–44. doi:10.1007/s11892-013-0375-y [published Online First:2013/03/16].   76. Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? European heart journal 2002:23(11):831–4. doi:10.1053/euhj.2001.3052 [published Online First:2002/06/04].   77. Cruz M, Maldonado-Bernal C, Mondragón-Gonzalez R, Sanchez-Barrera R, Wacher NH, Carvajal-Sandoval G, et al. Glycine treatment decreases proinflammatory cytokines and increases interferon-gamma in patients with type 2 diabetes. Journal of endocrinological investigation 2008:31(8):694–9. doi:10.1007/bf03346417 [published Online First:2008/10/15].  78. Arababadi MK, Nosratabadi R, Hassanshahi G, Yaghini N, Pooladvand V, Shamsizadeh A, et al. Nephropathic complication of type-2 diabetes is following pattern of autoimmune diseases? Diabetes research and clinical practice 2010:87(1):33–7. doi:10.1016/j.diabres.2009.09.027 [published Online First:2009/10/31].   79. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of clinical investigation 2006:116(6):1494–505. doi:10.1172/jci26498 [published Online First:2006/05/13].    80. Nagao K, Inoue N, Inafuku M, Shirouchi B, Morooka T, Nomura S, et al. Mukitake mushroom (Panellus serotinus) alleviates nonalcoholic fatty liver disease through the suppression of monocyte chemoattractant protein 1 production in db/db mice. The Journal of nutritional biochemistry 2010:21(5):418–23. doi:10.1016/j.jnutbio.2009.01.021 [published Online First:2009/05/09].   81. Xu H, Nakayama K, Ogawa S, Sugiura A, Kato T, Sato T, et al. Elevated plasma concentration of IP-10 in patients with type 2 diabetes mellitus. Nihon Jinzo Gakkai shi 2005:47(5):524–30. [published Online First:2005/09/01].  82. Esposito K, Pontillo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, et al. Association of low interleukin-10 levels with the metabolic syndrome in obese women. The Journal of clinical endocrinology and metabolism 2003:88(3):1055–8. doi:10.1210/jc.2002-021437 [published Online First:2003/03/12].   83. van Exel E, Gussekloo J, de Craen AJ, Frölich M, Bootsma-Van Der Wiel A, Westendorp RG. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes :the Leiden 85-Plus Study. Diabetes 2002:51(4):1088–92. doi:10.2337/diabetes.51.4.1088 [published Online First:2002/03/28].   84. Korea Institute of Oriental Medicine. Chenpi. Korean Intellectual Property Office 2007:https://doi.org/10.20929/KTKP.MED.0000084310.
85. Kim HW, Lee KS, Song BK. Study on the Effects of Gamibojungikgi Tang on Ovarian Reaction and Pregnancy in Obesity Mice. The journal of orienetal gynecology 2000:
86. Min BH, Lee IS. The Effect of Gamiyukgunjatang on the Obesity in Rats. The journal of orienetal gynecology 2000:13(1):469–84.
87. Bae IT, Jeong HW. The Effects of Yukgunja-tang on the Change of Weight and Serum levele in Mice Fed High Fat Diet. Korean J Oriental Physiology &Pathology 2003:17(6):1412–18.
88. Kim IJ, Cho JH, Jang JB, Lee KS. A case report of amenorrhea with obesity. Korean J. Korea Institue of Oriental Medical Informatics 2005:11(1):47–51.
89. Shin DG, Kim DK, Lee JY. A Clinical Study on the Effects of Gwakhyangjunggi-san gamibang on 9 Obest Children. J Pediatr Korean Med 2001:15(1):183–94.
90. Kim BJ. Effects of Leejung-tang, Rikkunshito, and Bojungikgi-tang on Transient Receptor Potential Vanilloid 4 Channels. J Korean Med Obes Res 2018:18(2):57–63.  91. Choi JS, Chang SK, Cho CS, Kim CJ, Han DU. Effects of Sopyung-tang Extract on Blood Glucose &Antioxidant Enzyme Activities of Streptozotocin-induced Diabetic Rats. Korean J Orient Int Med 2008:29(1):90–103.
92. Joo JH, Kim DW. The effects of Yukmijiwhangtangkamibang on diabetic rat induced by streptozotocin. Korean J Oriental Medical Prescription 2006:14(2):33–44.
93. Lee E, Ko H. Saenggangeonbi-tang's Effect on Alcoholic Liver Cirrhosis-1 Case. J Korean Oriental Med 2000:21(3):213–19.
94. Choi KH, Kim HY, Song IS. Two Cases Reports of Chlid Fatty Liver with Obesity. J Pediatr Korean Med 2008:22(3):95–103.
|
|












