I. Introduction
Along with drug-related hepatotoxicity, certain dietary supplements or folk herbs can induce the hepatic injury1. Among drug-induced liver injury (DILI), recently herb-induced liver injury (HILI) is getting attention due to the broad adoption of herbs worldwide2. One study reported that herbal drugs are responsible for 24.2% of DILI cases in China3.
On the other hand, especially in Korea, there are arguments that HILI has been overestimated and then exaggerated for the risk size of herb related hepatotoxicity4. One group reported that herbal drugs attributed 30.7% of 371 DILI-related hospitalizations in Korea5. However, another study presented the 0.5% proportion of HILI among 567 cases of the adverse drug reaction (ADR) on liver from nine regional pharmacovigilance centers6.
Korea has a long history traditionally to use the herbs for health and treatment of diseases. This fact has led to that many peoples administrate herbs by themselves without any consults to Korean doctors7. Furthermore, even medical doctors frequently don’t distinguish folk drugs taken by general subjects themselves from herbal medicines by prescribed by Korean Medicine doctors when they reported the HILI in scientific articles8. Recently, there are many concerns about the adverse reactions by functional food supplements in Korea9,10.
This case report presents a hepatotoxicity by Salvia Plebeia R. Br., a folk herb, used by many peoples for their respiratory symptoms. This report will inform Korean Medicine doctors and let them have attention the possibility of hepatic injury by folk herb including S. Plebeia in clinics.
II. Report of the case
1. Medical history and examination
A 58-year-old man, an engineer of pokeurein, has been healthy with a little obese body mass index (BMI, 26.2). He has enjoyed the drinking (1 bottle of soju daily) and smoking (1 pack daily), however he had not past or family history of hepatic disease. Recently, the patient experienced 5 kg of weight loss in 2 months, and felt malaise and anorexia, which led to hospitalization in a Hospital. He was diagnosed with alcoholic hepatic disorder through medical examination including abdominal sonograph and blood tests. The patient stopped both drinking and smoking, and discharged with normal condition after 7-day treatment.
He returned into his general work as an engineer of pokeurein. On 14 day of discharge from the hospital, he visited a Korean Medicine hospital with complaining the moderate level of nausea, dizziness and mild difficulties in speech and walking. He was hospitalized in a Korean Medicine hospital, with a suspected possibility of the electrolyte imbalance, brain infarction or Parkinson disease accompanying with chronic alcoholic liver disorder. From the laboratory tests, the abnormally lowed levels of sodium (112 mol/L, normal range 135-147 mol/L) and chloride (87 mol/L, normal range 95-110 mol/L) existed. In addition, slight elevated aspartate aminotransferase (AST, 63 IU/L) and total bilirubin (1.8 mg/ml) as well as the reduced platelet count (6.8×104) and serum albumin (3.0 g/dL) were found. In particular, his hepatic stiffness measurement (LSM, 35.8 kPa) showed a range of liver cirrhosis using Fibroscan. Some gas in bowel loops was observed from an x-ray examination for abdomen (Fig. 1).
2. Treatment and occurrence of hepatotoxicity
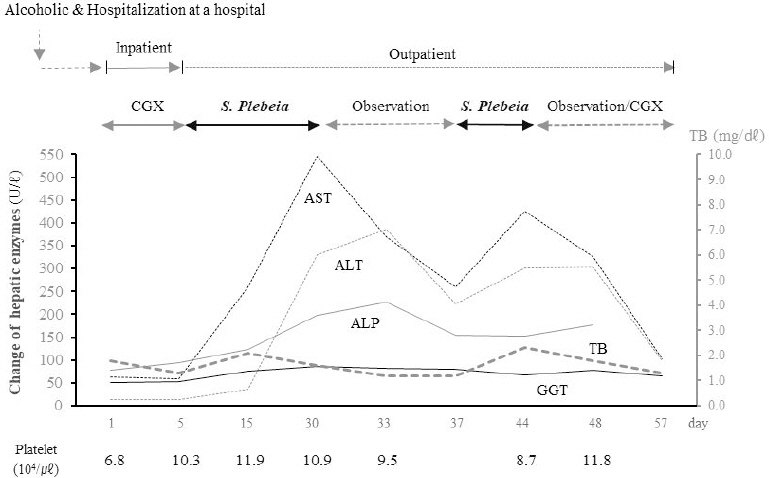
In order to correct the electrolyte imbalance, intravenous therapy was given to the patient, while an herbal medicine, Chungganplus syrup (CGX, two packs per day) was also prescribed for care hepatic disorder. His main symptoms such a s nausea, dizziness, and lagging speech and walking began to improve quickly during those treatments. Thus, the patient discharged after five-day hospitalization, and laboratory tests including electrolytes were normal range except a mild reduction of platelet count (10.3×104/μl).
When the patient visited my clinic department on 10 days later of discharge-hospital, as an outpatient, his chemistry test showed the abnormal elevation of AST up to 259 IU/L. This unexpected finding led me to recheck the hepatic enzymes which presented an acute severe hepatic injury, AST 546 U/L, ALT 330 U/L, ALP 198 U/L, and GGT 86 U/L, respectively. From the interview, S. Plebeia was suspected as the cause, which the patient drunk it (as boiled water) for his respiratory symptoms, coughing and mild sputum. After stopping the administration of S. Plebeia, the elevated hepatic enzymes were rapidly decreased by half-levels, and then those enzymes jumped up again in one week later. It was noticed that the patient re-administrated with S. Plebeia. In fact, the patient didn’t believe the possibility of hepatotoxicity by S. Plebeia, thus he retried to take S. Plebeia. The hepatic enzymes became almost normalized since the patient quit the drinking of S. Plebeia-boiling water for 2 weeks (Fig. 2).
During the hepatic injury happening, the patient didn’t feel any subjective discomfort symptom including fatigue or indigestion. The RUCAM score was 12, which met the criteria for toxic hepatitis by S. Plebeia. As same as inpatient treatments, only CGX had been prescribed for the patient.
III. Discussion and Conclusion
Salvia Plebeia R. Br. is widely distributed in many countries, especially in Asia. This plant has been used as a folk herb for the treatment of cough, inflammation and haemorrhoids11. Recent researches reported its biological activities such as antioxidant, antimicrobial and even hepatoprotective activities12-14. One group conducted a toxicological study of S. Plebeia mixed with red ginseng (ratio 3:1 as 30% ethanol extract) using 4-week oral administration in rats. This study demonstrated the safety of S. Plebeia as over 2,000 mg/kg bw/day of no-observed-adverse-effect level (NOAEL)15.
S. Plebeia is an edible plant, which can be taken by general population without any warning. However, the common materials known as safe can induce hepatic injury to certain subjects under certain conditions. In this case, although his hepatic inflammation was sedative on time point of admission, the patient has a reduced hepatic function due to cirrhotic change evidenced by LSM measurement, which was supported by reversed AST /ALT ratio (68 and 13 U/L, >3) and the reduced platelet count and serum albumin. This cirrhotic condition might increase the hepatic susceptibility against S. Plebeia as well as risk for the disturbance of electrolytes. The cirrhotic change indicates the notable impairment of hepatic capacity for drug metabolism16. Regarding the causality assessment, Roussel Uclaf Causality Assessment Method (RUCAM), an algorithm to measure the strength of association between hepatic injury and suspected agent, is commonly used. RUCAM score is considered as highly probable (>8), probable (6-8) or possible (3-5), and scores ≤3 were not included in HILI. Then, the score of this case was 12 as follows: 2 from “Time to onset-5 to 90 days”, 3 from “Course-decrease ≥50% within 8 days”, 2 from “Risk factor-alcohol and age ≥55 years”, 2 from “Exclusion of others causes” and 3 from “Response to re-administration” respectively.
In fact, there is the case report for serious liver and kidney damage due to the misuse of S. Plebeia17. The development of adverse reaction to drugs or food materials is decided by three factors; drug/food property itself, genetic background of individual and environmental factors including underlying disorders, concomitant drug, alcohol use, gender or age18. Hepatotoxicity is the most serious form of ADR or food-related adverse reaction. This case would belong to idiosyncratic metabolic responses that show the dose independent and unpredictable patterns19. The types of liver injury can be classified as hepatocellular (R≥5), cholestatic (R≤2), or mixed (2<R<5), based on the ratio of serum ALT to ALP designated as the R value ([ALT value/ALT UNL]/[ALP value/ALP UNL])20. Comparing to ALP and total bilirubin, both AST and ALT were remarkably elevated, and the R value was 5.4, which indicated the hepatocellular injury type. Contrast to Western drug-related hepatotoxicity, the portion of hepatocellular injury type is known to generally be predominant in HILI comparing to other types21. One study showed the similar clinical outcomes among three types of hepatic injuries22, while others presented the poor outcomes and higher mortality in patients with hepatocellular type of DILI23.
The principle of management for drug/food-related hepatic injury is to stop early the use of suspected agents. The vast majority of cases of DILI recover fully clinically and biochemically, however patients meet the criteria for Hy’s law, defined as either AST or ALT≥3×UNL and TB≥2×UNL without evidence of cholestasis (ALP≤2×UNL), shows the approximately 10 % of mortality24. The patient of this case was not meet the criteria for Hy’s law. He had showed the recurrence after the re-administration with S. Plebeia again because he didn’t accept the cause of S. Plebeia. However, the patient confirmed the no used of alcohol during of outpatient. He has been prescribed CGX and recovered finally laboratory tests near to normal range. CGX is 10 ml syrup containing 2 g extract from 13 herbs, and its detail composition has been described in previous report25. CGX has been used for both acute and chronic liver disorders including DILI based on its hepatoprotective effects26,27.
This case report presented S. Plebeia-induced hepatotoxicity. S. Plebeia has been commonly used as a fork remedy in Korea, thus Korean doctors need to be aware of it in clinical field.