I. Introduction
The neuronal cell damages cause variety spectrums of brain tissue damage related diseases. As a result of abnormal neuron injuries, brain tissue has been considerably gotten chances to incidence of variety diseases; represented by acute type of stroke, and neurodegenerations including Alzheimer’ disease, Parkinson’ disease, or Huntington’ diseases as well. Among the above them stroke is one of the most considerable disease in global public health. In addition, the rates of incidence of stroke also has been steadily increased1. For instance, cerebral ischemic stroke has been already reached up to approximately 80% of all patients with stroke2-4. Various etiological conditions have been suggested to etiological factors of stroke, mainly due to neuronal cell death, metabolic disturbance, excitotoxicity, and inflammatory response by either acute or chronic types5. Moreover, blood vessel occlusion owing to a thrombus, an immediated rack of supplements of oxygen and glucose in to the brain tissue, especially cerebral areas6,7. The above causes are also closely associated with the neuronal cell death by direct or indirect mode. Therefore, understanding the pathological mechanisms of stroke, which is focused on the neuronal cell death is the most crucial issue to develop clinical therapeutics.
Therefore, some of therapeutics have been developed for preventing from continuous neuronal cell injuries due to stroke, such as Extracellular Vesicles and Gasdermin proteins8,9. Up to date, the oxidative stress and inflammation mediated neuronal cell injury is well evidenced to understand pathophysiological progressing of stroke in the brain tissue. During suffering from stroke, reactive oxygen species (ROS), which is a harmful molecule of free radicals, easily accumulated and exclusively react to cell membranes, DNA, and mitochondrial. Because of the above abnormal reactions, mitochondrial dysfunction, DNA fragments, and the misfolding of proteins are happened10-12. Additionally, brain is a main tissue to damage by oxidative stress owing to relative less oxygen consumption, lack of antioxidant component, and plenty of polyunsaturated fatty acid. Therefore, to reduce oxidative stress and enhance antioxidant components are mainly focused on the therapeutic accesses against stroke.
On the other hand, Traditional Korean Medicine (TKM) has developed based on the clinical practice for thousand years. In TKM, stroke has been known to be caused by various Korean Medical etiologies such as “fire and heat” (火熱) “qi deficiency” (氣虛) “dampness and phlegm” (濕痰) and “blood stasis” (瘀血), and these days the stroke is mainly evoked by blockage of blood stream13. Recently, there are potent evidences well documented that herbal medicines are effective to treat stroke with pharmacological properties as well as correspond mechanisms12-16. Based on these recent clinical practice and studies of the TKM, a new herbal mixture has been prescribed including Gastrodia elata Blume, Codonopsis lanceolata (Siebold & Zucc.) Benth. & Hook. f. ex Trautv., Salvia miltiorrhiza Bunge, Curcuma longa L. (Zingiberaceae), and Astragalus mongholicus Bunge, (called, Chenmadansam- gamibokhap-tang, CDBT) which known as effective for ischemic stroke. Gastrodia elata Blume could reduce neuron cell apoptosis via reducing neuron cell damage by free radical, inhibiting Ca2+ influx into cells and decreasing the neuron toxicity by counteracting glutamate effect17. Curcuma longa L. and Codonopsis lanceolata play a protective role in brain injury through its anti-oxidant and anti-inflammatory activities18,19. Tanshinone IIA, a major component of Angelicae Gigantis Radix, has a neuronal protective effect by inhibiting the activity of caspase-3 after hypoxic nerve injury and has anti-inflammatory effects by decreasing the expression level of TNF-α and IL-1β20. Astragalus mongholicus Bunge can play an important role in improving the damage of ischemic brain tissue by increasing expression levels of VEGF and VEGF receptor-221. To investigate the pharmacological effects of CDBT and corresponded mechanisms, the CDBT was applied to protect hypoxia-induced neuronal cell death using mouse hippocampus neuronal cell line, HT-22 cells. In addition to explain the possible underlying mechanisms of CDBT oxidative stress and inflammation which were derived from microglial activation were also conducted in this study.
II. Material and method
1. Materials
1) Preparations of CDBT
The CDBT, which is a new prescribed herbal mixture with water extract, is composed of same amount of five different herbal plants including Gastrodia elata Blume, Codonopsis lanceolata (Siebold & Zucc.) Benth. & Hook. f. ex Trautv., Salvia miltiorrhiza Bunge, Curcuma longa L. (Zingiberaceae), and Astragalus mongholicus Bunge, respectively (Table 1). All herbal plants were obtained from the Dunsan Oriental Hospital of Daejeon University. The herbal mixtures were boiled with distilled water (DW) at 100 °C for 4 hrs and filtered with 300 mesh filter (50 μm). Condensing during 1hr of extraction, sample was placed under -70 °C for at least 3 hrs for the frozen extract processing. The frozen lyophilization was performed over than 72 hrs and sample was collected and weighed. The final yield was 8.96%.
Table 1
Components of CDBT and Its Ratio
2) Fingerprinting analysis of CDBT
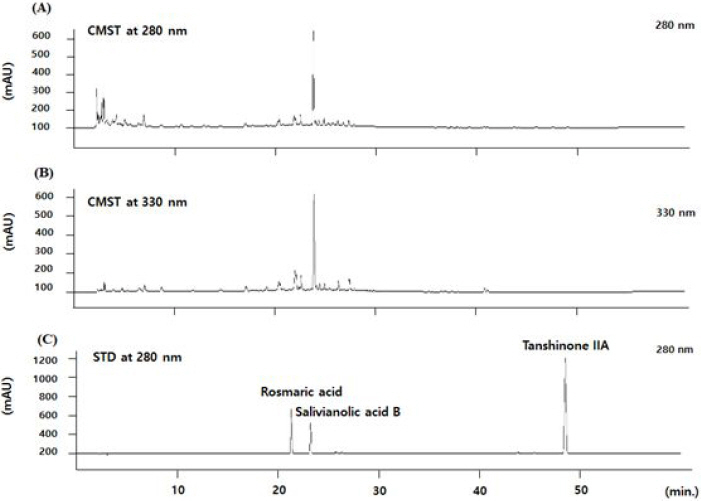
To identify the chemical features and reproducibility of the CDBT, fingerprinting analysis was performed using high-performance liquid chromatography-diode array detector-mass spectrometry (HPLC-DAD-MS) for either CDBT or its reference compounds; especially for Salvia miltiorrhiza Bunge with its well-matched chemical compounds including salvianolic acid B, rosmarinic acid and tanshinoe IIA, respectively. Briefly, after the dissolution (20 mg of CDBT and 0.01 mg of three reference chemicals in 1 mL 50% methanol) with filtration, and then these formulations were subjected to HPLC analysis of Agilent 1100 series. A Phenomenex Prodigy C18 (4.6×250 mm; particle size 5 μm) column was eluted with solvents A (10% acetonitrile in water containing 0.1% formic acid) and B (DW) at a flow rate of 0.4 mL/min. Solutions of 15% A and 85% B were changed to 60% B for 30 min, 40% B for 40 min, and 0% B for 60 min. The histograms and quantification analysis were obtained under the condition of 280 and 330 nm (Fig. 1 A to C).
Fig. 1
Histogram of CDBT and its major compounds analysis.
CDBT and its major chemical compounds were adapted to the HPLC-DAD-MS under the condition of UV wave length of 280 and 330 nm, to verify its chemical features or reproducibility. (A) Two-dimential histogram analysis of the CDBT under the 280 nm and (B) 330 nm. (C) The chemical compounds of the CDBT, rosmaric acid and salvianolic acid were also analyzed at the same condition of the CDBT under the 280 nm of wavelength condition.
2. Cell culture
Mouse derived hippocampal neuronal and microglial cell lines, such as HT-22 cells and BV-2 cells, were obtained from the Department of anatomy from Medical School of Chung-Nam-National University. The cells were cultured in DMEM with 10% FBS and antibiotics (100 U/mL penicillin G and 100 μg/mL streptomycin). The cells were maintained under humidified conditions at 37 °C in 5% CO2.
1) Cytotoxicity and cell proliferation analysis
Hypoxia condition was performed by oxygen and glucose deprivation/reperfusion (OGD/R) according to the previous methods using hypoxia chamber22. Briefly, the HT-22 cells (3×103) were seeded to 96-well microplates with 10% FBS containing DMEM. Cells were pre-treated with various dose of the CDBT without/with hypoxia condition. For cytotoxicity effects of CDBT, cells were incubated 48 hrs at concentrations given, and cell proliferation assay was performed under the condition of during 18 hrs hypoxia and 2 hrs recovery experiment.
2) Neuronal cell damage by hypoxia chamber
Neuronal cell damages were induced by OGD/R according to the previous methods22. HT-22 cells were cultured in a glucose-free DMEM, and then incubated with sealed air tight container. This condition resulted in a hypoxic atmosphere under the condition of absorbing oxygen and carbon dioxide productions. Then, cells were maintained in hypoxic conditions at 37 °C for 18 hours. After incubation, medium was discarded, and changed normal DMEM with glucose. Cells were further cultured for 2 hrs for re-oxygenation under normoxic condition to generate OGD/R. The cells cultured in growth culture medium under normoxic condition and served as a negative control through all hypoxic condition of neuronal cell experiment.
3) Microglia cells activation
In order to investigate the corresponded mechanisms of the CDBT, especially redox system homeostatis of neuronal cells and inflammation in microglial cells, BV-2 cells were activated by stimulation of LPS (0.1 μg/mL) or co-treatment with LPS (0.1 μg/mL) with IFN-γ (100 U/mL) treatments. The BV-2 cells were seeded to the 24 well-plates as a density of 2×105 cells/mL, then incubated for overnight at the 37°C, 5% CO2 condition. The CDBT (25, 50, and 100 μg/mL) were treated to the cells prior to 4 hours of LPS treatment. After LPS treatment, cells were further incubated 24 hours then measured NO and inflammatory cytokines from cell culture medium. For microglial activation mediated neuronal cell damages, the prepared conditional medium from co-treatments of LPS and IFN-γ were added to the HT-22 cell culture plates. After 18 hours of incubation, cellular oxidation and cell death signals were measured.
3. Biochemical analysis
1) Cellular oxidation analysis
Cellular oxidations of HT-22 cells with hypoxia condition or BV-2 cells activation was measured using CellROX® and dihydroethidium. Briefly, cells were seeded at a density of 5×105 cells in 60-mm of glass bottom dishes (Thermo) and treat the cells with the CDBT (50 and 100 μg/mL) or ascorbic acid (100 μg/mL). Dose-dependent modes of CDBT were treated to the dishes 4 hrs prior to hypoxia condition or LPS stimuli. After then, further 30 min incubation with final concentration of 5 μM of CellROX® reagent was added to the cells and medium was discarded A total 3 times of washing cells with PBS and fix with 3.7% formaldehyde for 15 min, thereafter a nuclear counterstain with hochest, then permeabilize the cells with 0.5% Triton X-100 for 10 min.
The cell permeable fluorogenic DHE was used to detect for the generations of superoxide radicals. A final concentration of 5 μM of DHE in fresh DMEM medium was added and dishes were further incubated in the dark condition for 30 min at the 37 °C. Then cells were washed with PBS at least two times.
After obtained all of stained sample, images were taken by fluorescence microscope (ZEIZZ). Images were analyzed using ImageJ free software. 200 cells of each samples were analyzed under the 630× magnification.
2) Analysis of cellular images
Cellular damages were captured appropriated images by performance of either immunohistochemistry (IHC) or immunofluorescence (IF) analysis. Briefly, the HT-22 cells were seeded at a density of 2.5×104 cells in 12 well plates and pre-treated with the CDBT (50 and 100 μg/mL) or ascorbic acid (100 μg/mL), respectively. Then cells were put under the condition of OGD/R hypoxia. After that cells were washed with PBS after entire removed medium and further incubated with 0.5% Triton X-100 for 10 min at RT. After incubation, the cells were fixed with 3.7% formaldehyde for 15 min, and with PBS two times. The cells were incubated using normal horse serum (2.5%) for 1 hrs at RT, then added primary antibodies against Hypoxia-inducible factor (HIF)-1α (1:100), cytochrome c(1:100), 4-hydroxynonenal (4-HNE, 1:200), 8-hydroxy-2’-deoxyguanosine (8-OHdG, 1:100) inducible nitric oxidase (iNOS, 1:100), Cu/Mn- superoxide dismutase (SOD, 1:100), and caspase-3/7 (1:100), were incubated under the 4 °C for overnight, respectively.
For IHC analysis in the present study, the 8-OHdG and 4-HNE in HT-22 with hypoxia condition were developed and avidin-conjugated secondary antibody which were incubated at RT for 2 hrs, then the signals were enhanced by development with 3,3’-diaminobenzidine (DAB). The positive signals were detected under the light microscopy condition.
For IF analysis, HIF-1α, cytochrome c, iNOS, Cu/Mn-SOD, 4-HNE, and caspase-3/7 were applied and fluorescence conjugated secondary antibodies (Green fluorescence for Aloxa 488 and red fluorescence for Aloxa 594) were added to slides (1:200 for each) and incubate 1 hr at RT. Then washed with PBST solution (0.5% Tween-20 in PBS) two times and PBS for one time. Nucleus counting staining was conducted with hochest and positive signals were obtained under the fluorescence microscopy condition.
Cell death signaling was detected using Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) assay. The positive signals were detected after application of DAB then captured images under the light microscopy condition (Olympus, ×200 magnification).
3) Measurement of nitric oxide (NO) contents
BV-2 cells were seeded at 24 well plates with intensity of 1×105 cells/mL. CDBT (25, 50, and 100 μg/mL) and ascorbic acid were pre-treated to the plates 4 hrs before LPS (100 ng/mL) treatment, respectively. Then, plates were incubated at 37 °C, 5% CO2 condition for overnight. The nitric oxide (NO) level in cell culture medium was determined using the Griess’ method23. The final product of purple azo dye t was measured at 540 nm using a UV spectrophotometer.
4) Measurement of pro-and anti-inflammatory cytokines
BV-2 cells were seeded at 24 well plates with intensity of 1×105 cells/mL. Various doses of CDBT and ascorbic acid were pre-treated to the plates 4 hrs before LPS (100 ng/mL) treatment. After LPS treatment, plates were incubated at 37 °C, 5% CO2 condition for overnight. The levels of tumor necrosis factor (TNF)-α, interlukin (IL)-1β, IL-6, and IL-10 levels in cell culture medium were measured using commercial ELISA kits according to the manufacturers’ instructions. The absorbance at 450 and 570 nm was measured using a spectrophotometer.
5) Cellular redox analysis
The biochemical analysis in the cellular levels were performed after obtained cell lysates which were prepared using commercial cell lysate reagents. The lipid peroxide levels were determined using thiobarbituric acid reactive substances (TBARS) as previously described24. The TBARS contents were displayed as a unit of μM malondialdehyde (MDA). Briefly, 50 μL of cell lysates or standard samples were added to 500 μL of 20% trichloroacetic acid (TCA) and then mixed with 200 μL of 0.67% thiobarbituric acid (TBA), followed by heating at 100 °C for 45 min, cooling on ice and vigorously vortexing with 800 μL of n-butanol. After centrifugation at 12,000× g for 15 min, the absorbance of the upper organic layer was measured at 520 nm using UV spectrophotometer and compared with a 1, 1, 3, 3-tetraethoxypropane (TEP) standard curve.
The total glutathione (GSH) content was determined as detection of total thiol contents25. Fifty of diluted cell lysate samples or total GSH was combined with 80 μL of a DTNB/NADPH mixture (10 μL of 4 mM DTNB and 70 μL of 0.3 mM NADPH) in a 96-well microplate. Next, 20 μL (0.06 U) of a GSH-reductase (GSH-Rd) solution was added to each well. The reactions were read under the 412 nm of wave.
Catalase activity was assayed followed by color reaction method as previously mentioned26. Briefly, 150 μL of phosphatase buffer (250 mM, pH 7.0), 150 μL of 12 mM methanol and 30 μL of hydrogen peroxide were mixed with 300 μL of the serum sample or standard solutions in a 13×100 mm test tube. The reaction was allowed to proceed for 10 to 20 min and was stopped by the addition of 450 μL of Purpald solution (22.8 mM Purpald in 2 N potassium hydroxide). The mixture was left for 20 min at 25 °C, followed by the addition of 150 μL of potassium periodate. The absorbance of the purple formaldehyde adduct was measured at 558 nm using a spectrophotometer.
6) Analysis of caspase-3 activities
The microglial cell activation mediated neuronal cell death was measured by caspase-3/7 activities using commercial kit (CaspACE™ Assay System, Colorimetric, Promega). Briefly, HT-22 cells were seeded to the 6-well plate as a density of 1×106 cells/well, then the CDBT (25, 50, and 100 μg/mL) or ascorbic acid (100 μg/mL) prior to 4 hours of conditional media treatment, and further incubated for 18 hrs. The procedures were followed to manufacture’ protocol.
4. Statistical analysis
All data are expressed as the mean±standard deviation (SD). Statistically significant differences between the groups were analyzed by one-way analysis of variance (ANOVA) followed by post hoc multiple comparison Fisher’s LSD t-test using the IBM SPSS statistics 20.0 (SPSS Inc. Chicago, IL, USA). Differences at p <0.05, p <0.01, or p <0.001 were considered statistically significant.
II. Results
1. Cytotoxicity effects of CDBT on HT-22 cells
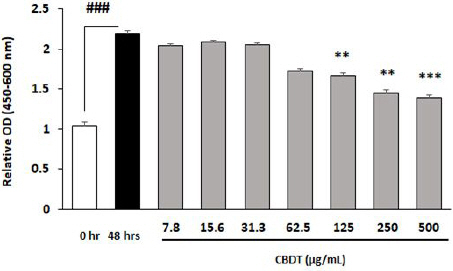
Various concentrations of CDBT (from 7.8 μg/mL to 500 μg/mL, as mode of serial dilutions) were treated to the HT-22 cells and incubated for 48 hours. Till the concentration of 62.5 μg/mL HT-22 cells were not influenced by the CDBT treatments, but since 125 μg/mL, CDBT showed cytotoxicity to the normal condition of HT-22cells (Fig. 2).
Fig. 2
Cytotoxicity effects of CDBT on the HT-22 cells.
HT-22 cells were seeded to the 96-well microplate at a density of 3×103 cells/well. After overnight of incubation, variety concentrations of CDBT were treated to the cells. The cytotoxicity assay was analyzed after further 48 hours of incubation with the CDBT. Incubation of overnight, various concentrations of CDBT were treated to the HT-22 cells and cytotoxicity was measured after 48 hrs incubation with CDBT. ###p<0.001 vs. 0hr, **p<0.01, and ***p<0.001 vs. Normal
2. Cell proliferation assay of CDBT on Hypoxia- induced HT-22 cells
To obtain the protective effects of CDBT against neuronal cell death, cell proliferation analysis under the hypoxia condition was performed. Since the dose of 32.5 μg/mL of the CDBT pre-treatment showed significant protect effects of the HT-22 cells from hypoxia condition. From this dosage to 250 μg/mL, pre-treatment with CDBT significantly deterred neuronal cell death against hypoxia condition (Fig. 3). Interestingly, the concentration at a 500 μg/mL also showed significant pharmacological properties, but not similar with 250 μg/mL dose.
Fig. 3
Cell proliferation assay of CDBT on the hypoxia-induced neuronal cell damage.
HT-22 cells were seeded to the 96-well microplate at a density of 1×104 cells/well. Various concentrations of CDBT were treated to the HT-22 cells 4 hours prior to hypoxia. After 18 hrs plates were move to reperfusion of oxygen supply then cell proliferation assay was measured. ###p<0.001 vs. control, *p<0.05, **p<0.01, and ***p<0.001vs. Hypoxia condition, respectively.

3. Effects of CDBT against hypoxia-induced neuronal cell death
Hypoxia condition, especially the method of OGD/R well induced HIF-1α in cellular levels. The programmed cell death signal molecule, cytochrome c was also correspondly increased depends on the HIF-1α expression which were evidenced by IF analysis (Fig. 4). Pre-treatment with CDBT, while notably reduced theses deterations of both HIF-1α and cytochrome c, respectively (Fig. 4).
Fig. 4
Effect of CDBT on the hypoxia-induced HIF-1α and cytochrome c.
HT-22 cells were seeded at a density of 5×105 cells in 60-mm glass bottom dishes and treat the cells with the CDBT (50 and 100 μg/mL) or ascorbic acid (100 μg/mL). Then cells were undergone OGD/R condition. Green fluorescence for detecting against HIF-1α using Alexa-488 conjugated secondary antibody (detect as green fluorescence) and red fluorescence for detecting against cytochrome c using Alexa-4594 conjugated secondary antibody (detect as red fluorescence), respectively. Immunofluorescence images were analyzed under the fluorescence microscopy circumstance (×630 magnification).

TUNEL assay well exhibited the neuronal cell death of the HT-22 cells by analysis of OGD/R (Fig. 5). Pre-treatment with the CDBT (50 and 100 μg/mL) obviously showed its anti-cell death efficacies on the hypoxia-induced neuronal cell death (Fig. 5). Ascorbic acid was used as positive control in this experiment, and showed similar effects on the HIF-1α, cytochrome c, and TUNEL assay.
Fig. 5
Effect of CDBT on the hypoxia-induced HT-22 cell death.
HT-22 cells were seeded at a density of 5×105 cells in 60-mm glass bottom dishes and treat the cells with the CDBT (50 and 100 μg/mL) or ascorbic acid (100 μg/mL). Then cells were undergone OGD/R condition. The cell death signals were obtained by performance of TUNEL assay.
4. Effects of CDBT against hypoxia-induced neuronal cell injury
IF analysis of a potent cell death related molecules, cleaved-caspase-3 positive signals were drastically enhanced owing to OGD/R of hypoxia condition (Fig. 6). On the other hand, pre-treatment with the CDBT promptly reduced the positive signals. Ascorbic acid was used as positive control in this experiment.
Fig. 6
Effect of CDBT on the hypoxia-induced neuronal cell death.
HT-22 cells were seeded at a density of 5×105 cells in 60-mm glass bottom dishes and treat the cells with the CDBT (50 and 100 μg/mL) or ascorbic acid (100 μg/mL). Then cells were undergone OGD/R condition. The positive signals of cleaved caspase-3 was detected by Alexa-594 conjugated secondary antibody. Immunofluorescence analysis under the fluorescence microscopy circumstance (×630 magnification).
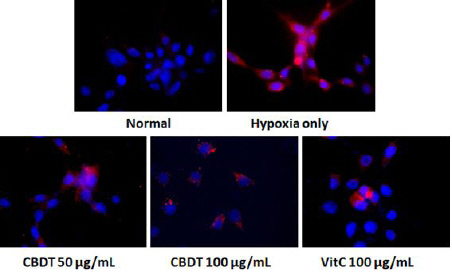
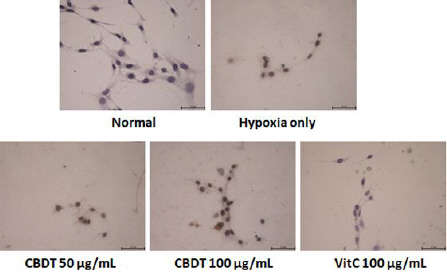
5. Effects of CDBT against hypoxia-induced DNA fragmentation
Fig. 7 displayed that the pharmacological effects of CDBT on the DNA fragmentation by OGD/R of hypoxia condition. Pre-treatment with the CDBT, however dramatically attenuated DNA fragmentations. Ascorbic acid was used as positive control in this experiment.
Fig. 7
Effect of CDBT on the hypoxia-induced DNA fragmention of HT-22.
HT-22 cells were seeded at a density of 5×105 cells in 60-mm glass bottom dishes and treat the cells with the CDBT (50 and 100 μg/mL) or ascorbic acid (100 μg/mL). Then cells were undergone OGD/R condition. The positive DNA fragmentation signals of 8-OHdG were obtained by IHC analysis.
6. Effects of CDBT against hypoxia-induced neuronal cell oxidation
1) IF analysis of 4-HNE
The final product of cellular oxidation was measured by IF analysis of 4-HNE, which is a potent marker of lipid peroxidation. Positive signals of the 4-HNE was strongly enhanced by hypoxia, whereas pre-treatment with the CDBT notably reduced the 4-HNE signals (Fig. 8). Ascorbic acid was used as positive control in this experiment.
Fig. 8
Effect of CDBT on the hypoxia-induced neuronal cell oxidation.
HT-22 cells were seeded at a density of 5×105 cells in 60-mm glass bottom dishes and treat the cells with the CDBT (50 and 100 μg/mL) or ascorbic acid (100 μg/mL). Then cells were undergone OGD/R condition. The final product of cellular oxidation, 4-HNE, was detected by Alexa-488- conjugated secondary antibody. Immunofluorescence images were analyzed under the fluorescence microscopy circumstance (×630 magnification).

2) Cellular oxidations assays
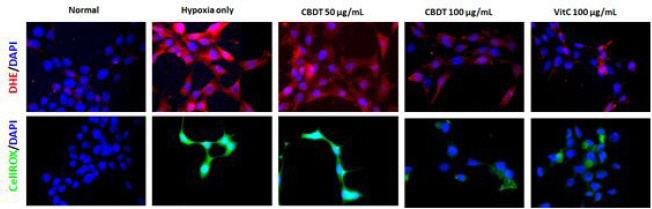
Whether the ROS led to mediate neuronal cell injury or not, cellular oxidation analysis was performed by both CellROX and DHE stainings. Hypoxia caused drastic increases of both CellROX and DHE signals, while pre-treatment with the CDBT decreased those of abnormal enhanced signals (Fig. 9). Ascorbic acid was used as positive control in this experiment.
Fig. 9
Cellular oxidations assays.
HT-22 cells were seeded at a density of 5×105 cells in 60-mm glass bottom dishes and treat the cells with the CDBT (50 and 100 μg/mL) or ascorbic acid (100 μg/mL). Then cells were undergone OGD/R condition. Cellular oxidations were performed using DHE for detecting superoxide radicals (Red fluorescence, upper panel), and CellROX dye (For green fluorescence, bottom panel). Images were captured under the fluorescence filter equipped microscopy condition (×630 magnification).
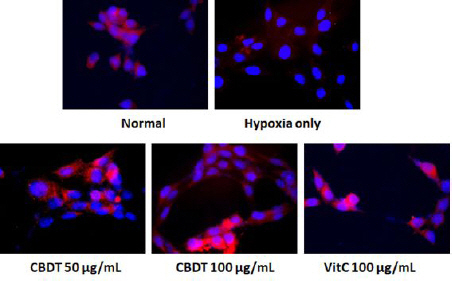
7. Anti-Oxidant Effects of CDBT on the hypoxia- induced neuronal cell oxidation
To determine anti-oxidant effect of CDBT against hypoxia-induced neuronal cell injury, IF analysis of Cu/Zn-SOD was performed. Pre-treatment with CDBT displayed its pharmacological properties by drastically increases of Cu/Zn-SOD positive signals. On the other hand, hypoxia condition severely deterred the positive signals of Cu/Zn-SOD (Fig. 10). Ascorbic acid was used as positive control in this experiment.
Fig. 10
Effect of CDBT on the hypoxia-induced neuronal cell oxidation.
HT-22 cells were seeded at a density of 5×105 cells in 60-mm dishes and treat the cells with the CDBT (50 and 100 μg/mL) or ascorbic acid (100 μg/mL). Then cells were undergone OGD/R condition. Cu/Zn-SOD was detected by IF analysis under the fluorescence microscopy circumstance (×630 magnification).
8. CDBT ameliorates microglia cells activation mediated neuronal cell damage
1) Effects of CDBT on oxidation by BV-2 cell activation
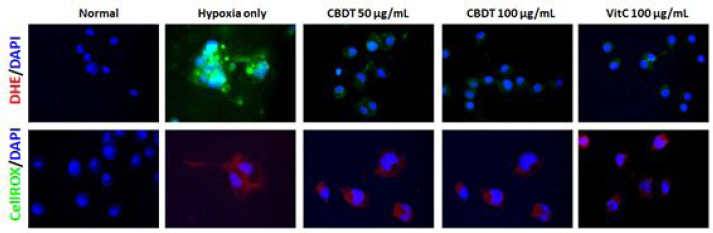
As comparing to the normal group, LPS (100 ng/mL) caused higher increases of CellROX® positive signals, while pre-treatment with the CDBT slightly decreased those of signals with LPS treated group (Upper panel of Fig. 11). To explain the activation of inflammatory response and oxidative stress from microglial cells, BV-2 cells were treated to the LPS (100 ng/mL) for microglial cells activation. LPS (100 ng/mL) considerably increased the DHE intensity as compared with normal group, whereas CDBT slightly decreased those of ROS generations as compared with LPS treated group (Bottom panel of Fig. 11). Ascorbic acid was used as positive control in this experiment.
Fig. 11
Effects of the CDBT on the LPS-induced BV-2 cell activation.
BV-2 cells were seeded at a density of 5×105 cells in 60-mm dishes and treat the cells with the CDBT (50 and 100 μg/mL) or ascorbic acid (100 μg/mL). Then cells were treated LPS (100 ng/mL) for overnight (18 hrs). Cellular oxidation that was from microglial cells activations were detected by CellROX® analysis. Images were captured under the circumstance (×630 magnification).
2) Effects on the microglial activation mediated neuronal cell injuries
To investigate the corresponded mechanisms of the CDBT on the neuronal cell death, microglial activation and its mediated neuronal cell damages were determined. Pre-treatment with the CDBT considerably reduced NO levels in the medium, as compared with LPS only group (Fig. 12-A). Additionally, treatment with thee conditional medium led to abnormal increases of TBARS contents as compared with normal group, while pre-treatment with the CDBT significantly ameliorated that of abnormal TBARS levels (Fig. 12-B).
Fig. 12
Effects of CDBT on Redox signals.
The conditional medium which was from BV-2 activation was added to the HT-22 cells for 18 hours, after pre-treatment of CDBT (25, 50, and 100 μg/mL) or ascorbic acid (100 μg/mL). After completion of incubation under the 37 °C, 5% CO2 condition. NO levels were measured in the BV-2 cell with LPS treatment condition of medium levels (A). Other parameters including TBARS (B), total GSH contents (C), and catalase activities (D) were determined in the cellular protein levels of HT-22 cells which were damaged by conditional media treatment. Data were expressed as mean±SD. ##p<0.01, ###p<0.001 vs. Normal, *p<0.05, **p<0.01, and ***p<0.001 vs. Conditional medium, respectively.

As a potent antioxidant component, total GSH was sharply decreased due to hypoxia condition, and catalase which is one of the most outstanding antioxidant enzymes were also deterred its activities by hypoxia condition. Pre-treatment with the CDBT significantly exerted to prevent from these abnormal deteriorations (Fig. 12-C, D). Ascorbic acid which was used as a positive control in this study showed similar effects.
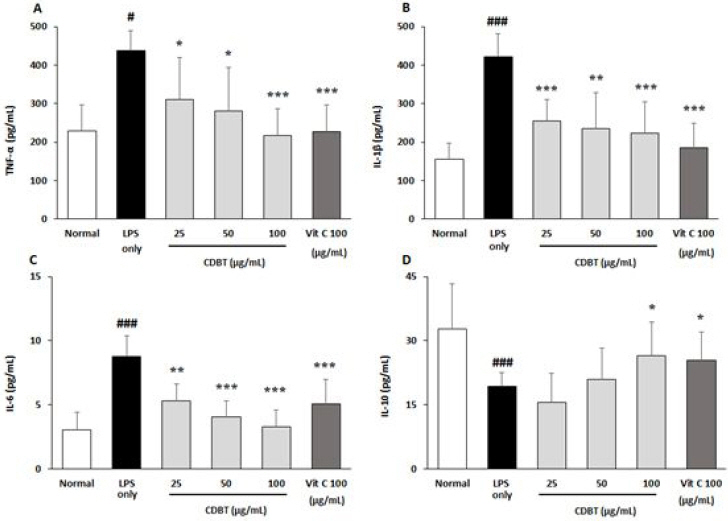
3) Effects on the microglial active inflammatory reactions
Next experiment was performed to investigate the pharmacological properties of the CDBT on microglial cell activation. LPS was treated to the BV-2 cells after 4 hours the CDBT or ascorbic acid. The LPS only group showed significant increases of TNF-α levels in medium as compared with normal group, whereas pre-treatment with the CDBT significantly lowered the elevated NO levels as compared with LPS only group (Fig. 13-A). Other pro-inflammatory cytokines, such as IL1-β and IL-6 were also significantly higher than that of normal group, but decreased these elevated cytokine levels in the pre-treatment with CDBT groups as compared with LPS only group (Fig. 13-B, C). On the other hand, the pre-treatment with CDBT significantly exerted to prevent from deletion of IL-10, which is an anti-inflammatory cytokine against LPS treatment (Fig. 13-D). Pre-treatment with ascorbic acid showed similar effects of the CDBT.
Fig. 13
Effects of CDBT on pro- and anti-inflammatory cytokines.
The conditional medium which was from BV-2 activation was added to the HT-22 cells for 18 hours, after pre-treatment of CDBT (25, 50, and 100 μg/mL) or ascorbic acid (100 μg/mL). After completion of incubation under the 37 °C, 5% CO2 condition. After completion of incubation the supernatant of cell culture medium was collected and inflammatory cytokines including TNF-α (A), IL-1β (B), IL-6 (C), and IL-10 (D) in the medium levels were measured by ELISA method. Data were expressed as mean±SD. #p<0.5, ###p<0.001 vs. Normal, *p<0.05, **p<0.01, and ***p<0.001 vs. Conditional medium, respectively.
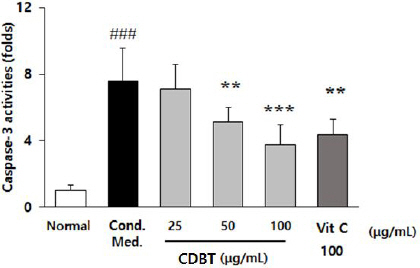
9. Effects on the microglial cell activation mediated neuronal cell damages.
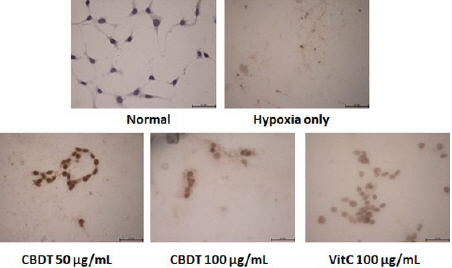
To verify the pharmacological effects of CDBT on the neuronal cell death owing to microglial cell activation, conditional medium was prepared by treated with LPS (100 ng/mL) and IFN-γ (100 U/mL) to the BV-2 cells. Conditional media exerted to significantly elevate caspase-3 activities in the HT-22 as compared with normal group (p<0.001), whereas pre-treatment with CDBT significantly decreased those higher alterations of caspase-3 activities as compared with conditional media only treated group (Fig. 14).
Fig. 14
Effects of CDBT on the neuronal cell death by microglial cells activation.
Conditional medium of microglial cell activation by treatment with LPS (100 ng/mL) with IFN-γ (100 U/mL). HT cells were splited to the 6 well-plate as a density of 1×106 cells/mL and pre-treatment with CDBT (25, 50, and 100 μg/mL) or ascorbic acid (100 μgmL) during 4 hrs before adding to conditional media treatment. Then plates were incubated to the cell incubator (37 °C, 5% CO2 supplement condition). After 18 hrs incubation, cell lysates were obtained, and caspase-3 activities were measured. Data were expressed as mean±SD. ###p<0.001 vs. Normal, **p<0.01, and ***p<0.001 vs. Conditional medium, respectively.
IV. Discussion
To date, there are many of etiological factors participate to accomplish hypoxia including oxidative stress, inflammation, and limitation of nutrition supplement. In addition, the hypoxia is complexly associated with various neuronal disorders regardless of acute or chronic types. This disease condition only exerted to the acute type of brain tissue damage such as stroke, but also caused neurodegenerations such as Alzheimer’s diseases, Parkinson’s diseases, or Huntington’ diseases27. Regarding the stroke, especially, it ranks as a second cause of death in the world. In addition, recent evidences well reported some of risk factors such as obesity, hypertension, smoking, abnormal diet habit, and lack of physical activities are caused incidence of stroke28,29.
Although the pathophysiological characters of hypoxia still remain unclear in recent days, but neuronal cell damages from oxidative stress and inflammation would thought generally up to date30,31. Additionally, the neuronal cell injuries due to microglial cell activation was also focused on to deeply understand hypoxia related neuronal diseases32,33. Thus, many of novel therapeutics on the hypoxia has been considered to modulate both oxidative stress and inflammation.
On the other hand, the TKM has been developed various treatment ways to treat hypoxia related disease, especially stroke with acupunctures and herbal medicines. Among the herbal medicines, some of previous studies well evidenced that they would useful to treat stroke by hypoxia. However, it is still needed to solve the underlying corresponded mechanisms of properties. Therefore, this study was performed to access the possibility using of herbal medicine to treat hypoxia-related stroke34-36. Based on the accumulated clinical practice, the herbal prescription of the CDBT was invented and applied mouse derived neuronal cell line, HT-22 cells under the hypoxia condition.
In the present study, firstly observed the possible cytotoxicity dose, and up to 125 μg/mL dosage of the CDBT, it didn’t show the cytotoxicity in the normal cell culture condition and showed anti-cell death efficacies against the hypoxia condition as well (Fig. 2 and 3). According to these results, in the present study was performed the effective dose of the CDBT as 25, 50, and 100 μg/mL. In addition, the neuronal cell of HT-22 model in the present study by OGD/R of hypoxia condition was well worked. Hypoxia only group considerably enhanced the positive signal (Part of green fluorescence, HIF-1α), and pre-treatment with the CDBT drastically blocked that of abnormal increases of HIF-1α signals in the HT-22 cells. Additionally, the cell death signals of cytochrome c release were also well correlated to the HIF-1α, but not pre-treatment with the CDBT (Fig. 4).
The oxidative stress and its related cell death signals were considerably enhanced by evidence of 4-HNE staining as well as TUNEL assay (Fig. 5 and 8). Additionally, the neuronal cell injury due to severe oxidative stress in the hypoxia condition was also considerably increased cell death signals such as releasing of cytochrome c as well as cleaved caspase-3 (Fig. 6). The DNA fragmentation, which is a corresponding potent marker of both cell death and oxidation, was also severely evoked concurrently as an evidence of 8-OHdG IHC analysis (Fig. 7). Pre-treatment with the CDBT also exerted to reduce the abnormal enhancement of the cellular oxidations owing to abnormal increases of ROS signals which were detected by CellROX and DHE (Fig. 9). These pathological alterations were significantly prevented from pre-treatment with the CDBT. Regarding antioxidant effects of the CDBT, it considerably prevented from the notable depletion of the Cu/Zn-SOD signals under the hypoxia condition (Fig. 10).
Particularly, brain tissue is well known for vulnerable organs to oxidation owing to high rate of oxygen consumptions, full of polyunsaturated fatty acids, easily transited metal irons, and sensitivity of blood-brain endothelial cells37-39. In addition, another cell types of brain tissue, such as either amygdala or microglials are also deeply related to the progression of neuronal cell damages40. Thus, next experiment was performed for focusing on the microglial cell activation-induced neuronal cell death. Conditional media was prepared by co-stimuli condition of the LPS with IFN-γ in the BV-2 cells, then treated to the HT-22 cells.
Evidenced by the NO levels in the media and cellular oxidations (Fig. 11 and 12-A) due to the microglial cell activations. All of results towards to the microglial cell activation-induced also concurrently arose which were evidenced by increases of TBARS, depletion of total GSH content, and deteriorations of catalase activities, respectively. Pre-treatment with the CDBT, however, significantly prevented most of the above abnormalities (Fig. 12-B to D). Particularly, pro-and anti-inflammatory cytokines were also normalized by pre-treatment with the CDBT as well (Fig. 13-A to D). Neuronal cell death was happened by microglial cells activation and the CDBT efficiently prevented from it as shown by caspase-3 activities.
Interestingly, the most chemicals such as rosmarinic acid and salvianolic acid were in Salvia miltiorrhiza Bunge. from the CDBT. This herbal plants were most popularly used to treat blood stream blood stream-related dysfunction such as “dampness and phlegm” (濕痰) and “blood stasis” (瘀血)41,42. Recent accumulated studies also well documented that the pharmacological properties of Salvia miltiorrhiza Bunge. in various brain diseases43-45.
Taken together, the CDBT showed protective effects on the hypoxia-induced neuronal cell damages and the underlying mechanisms were related to the relieving of oxidative stress mediated cell death signals.