I. Introduction
Insomnia disorder in patients with neurological disease reduces the patient’s quality of life1,2, and it is one of the main factors that adversely affects the overall prognosis by interfering with efficient daytime rehabilitation3. Various hypnotics are used to treat insomnia disorder; however, these medications can cause daytime hypersedation, which can lead to complications such as falls4, decreased cognitive function3,5, and parkinsonism6 that lead to poor prognosis and require new treatment options.
Ongyeong-tang (OGT) is a herbal prescription that originates from the “Synopsis of Prescriptions of the Golden Chamber” and has been used for menopausal symptoms, uterine diseases, skin diseases, insomnia, and other neuropsychiatric symptoms. Currently, the Korean Ministry of Food and Drug Safety recognizes the following indications for OGT extract: “The following symptoms of patients with burning hands and feet and dry lips: menstrual irregularities, dysmenorrhea, leukorrhea, menopausal disorders, insomnia, nervousness, eczema, cold sensation in the lower extremities, and frostbites”7.
However, this indication is based on contents from many previous medical classics and not from clinical studies. In fact, evidence related to menopausal symptoms8,9 and various female reproductive diseases10,11 is often reported in relation to OGT; however, no studies exist that evaluated its efficacy in insomnia.
Therefore, in this study, to explore the possibility of developing new treatment options for insomnia disorder in patients with neurological diseases, we retrospectively evaluated the efficacy and safety of OGT extract for insomnia disorder in patients with neurological diseases based on the medical records of one medical institution and examined the possibility of further research in patients.
II. Methods
1. Study participants
In this study, medical records of patients who visited Kyung Hee University Korean Medicine Hospital from January 1, 2021 to June 30, 2022 and received outpatient or inpatient treatment, meeting the following inclusion criteria and exclusion criteria, were included in this study. This study was approved by the Institutional Review Board of Kyung Hee University Korean Medicine Hospital (KOMCIRB 2022-06-004).
1) Inclusion criteria
(1) Patients diagnosed with stroke (I63, I61, I60), Parkinson’s disease and Parkinson’s syndrome (G20, G22), or Alzheimer’s disease (G309) confirmed via brain computed tomography or magnetic resonance imaging, or a combination of both
(2) Patients aged 19 years or older who complained of at least one type of insomnia disorder (sleep onset latency insomnia [SOL-insomnia], wake after sleep onset insomnia [WASO-insomnia], and early morning awakening insomnia [EMA-insomnia])12
(3) Patients taking herbal medicine, OGT extract (Sponsor: JEIL PHARMACEUTICUAL CO. LTD., Manufacturer: HANSOL PHARM. CO. LTD.)7 for treating insomnia disorder (Table 1)
Table 1
Composition of OGT Extract
(4) At least two instances where changes in insomnia symptoms can be measured (Korean version of the Pittsburgh Sleep Quality Index [PSQI-K])13 before and after prescribing OGT extract, with at least 7 days between each measurement
2. Review and investigation of medical records
Reviewing the medical records of patients who satisfied both inclusion and exclusion criteria, demographic sociological information was collected such as age and sex, types of insomnia disorder (SOL-insomnia, WASO-insomnia, and EMA-insomnia)12, information on underlying neurological diseases (stroke, Parkinson’s disease or Parkinson’s syndrome, or Alzheimer’s disease), information on OGT extract (dosage, frequency, total days taken, adverse side effects, etc.), medications other than OGT extract taken for insomnia disorder, and changes in insomnia symptoms.
The specific survey method for each survey item is as follows.
1) Whether the subjective symptoms of insomnia have improved
Improvement in insomnia symptoms was examined in medical records at least 7 days after the start of OGT extract administration as confirmed in the medical records. Based on the contents of the medical records, they were classified into valid, invalid, and deteriorating.
2) PSQI-K13
The PSQI-K score at the beginning of OGT extract administration confirmed in the medical records was defined as “PSQI-K before treatment”, and the PSQI-K score after 1 week of OGT extract administration was defined as “PSQI-K after treatment”. The PSQI score usually assesses symptoms in the “last 1 month” but was changed to “last 2 days” for the participants in this study. PSQI-K scores were examined for both total and detailed scores and outcomes were compared before and after treatment.
3) Use of sleep aid medications before and after OGT extract use
The use of concomitant sleep-related medications during OGT extract administration was identified and recorded. If other medications were used together to control insomnia, data of the names of the medications used, the duration of use, and the number of administrations per day were collected.
4) Pattern identification questionnaire results prior to OGT extract administration
The results of pattern identification questionnaires were examined to differentiate differences in the treatment effects according to types of pattern identification. The pattern identification questionnaire was developed for patients with acute stroke, which contains pattern identification formulas14 to examine the results. Based on these results, the probabilities of fire-heat, phlegm-dampness, Yin deficiency, and Qi deficiency were determined.
5) Details of OGT extract administration and adverse side effects
Instructions on how to administer the OGT extract were examined. The data examined included dose per administration (pack), number of administrations per day (number), and total duration (days). The medical records were also reviewed for any adverse reactions during the intake of the OGT extract, and details were included where an adverse reaction was confirmed.
3. Data and statistical analyses
Data such as sex were expressed as “Number (N)” and “percent (%)” or “Number (N)” and “Quartile” depending on normality, and continuous data (e.g., age) were summarized as average, standard deviation or median, skewed distribution, and minimum and maximum values depending on normality.
To assess the effect of the OGT extract on insomnia, we evaluated the improvement in subjective symptoms before and after treatment, including changes in the PSQI-K score. Improvement in the subjective symptoms was expressed as “Number (N)” and “percent (%)” or “Number (N)” and “quartile” depending on whether or not it was normal, and the change in the PSQI-K score before and after treatment was tested for statistical significance using the paired t-test if it showed a normality distribution and the Wilcoxon signed rank test if the p-value was <0.05.
In addition, to compare the characteristics of OGT extract responders and non-responders for insomnia disorder based on PSQI’s minimal clinically important difference (MCID) of 3 points, OGT responders (3 points or more improvement before and after treatment) and OGT non-responders (less than 3 points improvement before and after treatment) were divided according to the amount of change in the PSQI-K scores before and after treatment, demographic characteristics, comorbidity prevalence, prevalence of underlying neurological disease, concomitant use of sleep-related medications, and probability of pattern identification (fire-heat, phlegm-dampness, Qi deficiency and Yin deficiency). Nominal variables were tested using chi-square test or Fisher’s exact test (for non-normality distributions) depending on normality, and for continuous variables, t-test or Mann-Whitney U test (for non-normality distributions) were performed according to normality. Statistical significance was set at a p-value <0.05.
III. Results
1. Baseline characteristics of the research participants
From January 1, 2021 to June 30, 2022, a total of 26 patients with neurological diseases (cerebrovascular disease, Parkinson disease, Parkinsonism, or Alzheimer’s disease) received OGT extract for insomnia disorder, of which 24 satisfied the inclusion criteria. Two patients were excluded from the analysis because subjective symptom improvement of insomnia or assessment of the PSQI-K score was not present in the medical records (Fig. 1).
Fig. 1
Flow diagram of the present study.
OGT: Ongyeong-tang, PSQI-K : the Korean version of the Pittsburgh sleep quality index

The mean age of the 24 patients was 70.29± 9.63 years, with females comprising 58.3%. The most common type of insomnia disorder was the SOL-insomnia with 14 patients (58.3%), followed by the WASO-insomnia (10 patients, 41.7%). Cerebral infarction was the most common underlying neurological diseases with 14 patients (58.3%), followed by cerebral hemorrhage, Parkinson’s disease, and Alzheimer’s disease. Among the comorbid diseases, hypertension was the most common with nine patients (37.5%) (Table 2).
Table 2
Baseline Characteristics
2. Treatment details
The OGT extract administered to the participants in this study was in yellow-brown granules, corresponding to 3.0 g in one dose (one pack), and the herbs contained and dosages are indicated in Table 1. Generally, one pack is taken 1-3 times a day, but two packs may be taken once to enhance the effect. Of the participants in this study, 12 (50%) took one pack three times daily, and five (20.8%) took one pack twice daily. The remaining seven took one dose before bedtime, four took two packs once to increase effectiveness, and three took one pack of the original dose (Table 3).
Table 3
Detailed Medication Information
If patients in this study took other medications for insomnia disorder, took OGT extract in addition to their current medications, and did not take any other medications for insomnia disorder, no additional treatment was given other than an additional dose of the OGT extract. Out of the total 24 patients, eight patients (33.3%) took the OGT extract with hypnotics, with clonazepam as the most common hypnotic used followed by quetiapine (Table 3).
The duration of OGT extract administration for insomnia disorder was 14.42±9.2 days.
3. Evaluation of the effectiveness of OGT extract on insomnia disorder
1) Subjective symptoms of insomnia
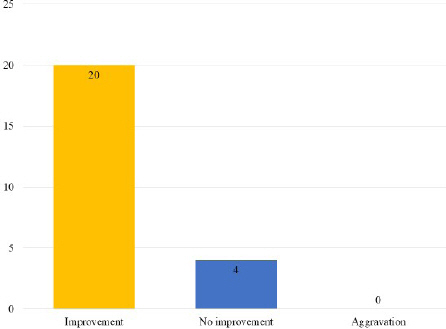
After an average of 14.42±9.2 days of OGT extract intake, 20 patients (83.3%) experienced improvement (valid). The remaining four (16.7%) noted no change in their symptoms (invalid) and none demonstrated worsening of symptoms (Fig. 2).
2) Changes in the PSQI-K score13
The total PSQI-K score decreased from 13.08± 4.54 before OGT extract administration to 10.42 ±4.58 after administration, showing a statistically significant improvement (p<0.001, Table 4). Scores for each subcategory also showed an improvement, with sleep quality (C1), sleep duration (C2), sleep disturbance (C5), use of sleeping medications (C6), and daytime dysfunction (C7) showing statistically significant improvements (Table 4).
Table 4
Korean Version of the Pittsburgh Sleep Quality Index Score before and after OGT Extract Administration
4. Comparison of characteristics of patients who responded and failed to respond to OGT extract
There were 10 patients (responders) who were deemed to have clinically significant improvement with a PSQI score of 3 or more points (responders), and the remaining 14 patients were considered as non-responders. There were no statistically significant differences in the demographic and sociological characteristics such as age, sex, type of insomnia, type of underlying neurological diseases, and prevalence of other comorbidities. The combination of hypnotics also did not have a significant effect on clinical efficacy.
The probability of pattern identification also did not show a statistically significant difference between the two groups. However, in the pattern identification type, except for Qi-deficiency, both groups showed similar probability tendencies (both negative and positive probabilities). The probability of Qi-deficiency was calculated as a negative probability in the improvement group and a positive probability in the no improvement group, showing a high probability of Qi-deficiency in the no improvement group.
5. Occurrence of adverse side effects
None of the 24 participants complained of adverse reactions.
Table 5
Difference in Pattern Identification Probability according to Clinical Symptom Improvement
| Improvement group (n=10) | No improvement group (n=14) | p-value | |
|---|---|---|---|
| Age, years | 70.80±10.25 | 69.93±9.53 | 0.835* |
|
|
|||
| Sex | |||
| Male, number (%) | 5 (50.0) | 5 (35.7) | 0.389# |
| Female, number (%) | 5 (50.0) | 9 (64.3) | |
|
|
|||
| Subtypes of insomnia | |||
| SOL-insomnia, number (%) | 6 (60.0) | 8 (57.1) | 0.611# |
| WASO-insomnia, number (%) | 4 (40.0) | 6 (42.9) | 0.611# |
| EMA-insomnia, number (%) | 0 (0.0) | 0 (0.0) | |
|
|
|||
| Underlying neurological diseases | |||
| Cerebral infarction, number (%) | 6 (60.0) | 8 (57.1) | 0.611# |
| Cerebral hemorrhage, number (%) | 0 (0.0) | 4 (28.6) | 0.094# |
| Parkinson’s disease, number (%) | 3 (30.0) | 1 (7.1) | 0.178# |
| Alzheimer’s disease, number (%) | 1 (10.0) | 1 (7.1) | 0.670# |
|
|
|||
| Other underlying diseases | |||
| Hypertension, number (%) | 3 (30.0) | 6 (42.9) | 0.418# |
| Diabetes mellitus, number (%) | 0 (0.0) | 2 (14.3) | 0.330# |
| Dyslipidemia, number (%) | 3 (30.0) | 5 (35.7) | 0.561# |
| Ischemic heart disease, number (%) | 0 (0.0) | 1 (7.1) | 0.583# |
| Atrial fibrillation, number (%) | 1 (10.0) | 2 (14.3) | 0.629# |
| Depression, number (%) | 0 (0.0) | 1 (7.1) | 0.583# |
| Spinal stenosis, number (%) | 0 (0.0) | 1 (7.1) | 0.583# |
|
|
|||
| Combination of hypnotics, number (%) | 6 (60.0) | 8 (57.1) | 0.611# |
|
|
|||
| Probability of pattern identification | |||
| Fire-heat, average±SD | 2.03±1.87 | 1.71±2.16 | 0.706* |
| Phlegm-dampness, average±SD | -12.14±2.32 | -11.82±2.97 | 0.770* |
| Qi-deficiency, average±SD | -0.45±1.53 | 0.16±1.54 | 0.345* |
| Yin-deficiency, average±SD | -5.86±1.17 | -6.39±1.05 | 0.271* |
IV. Discussion
OGT, which was evaluated in this study, first appeared in the “Synopsis of Prescriptions of the Golden Chamber” published during the Han dynasty period in China, and is one of the longest prescribed herbal medicines in East Asia16. At the time, the prescription was recommended for genital bleeding and burning sensation in menopausal women, infertility in women of childbearing age, and menstrual abnormalities. Over the years, as documented in the medical classics, it is not only used for gynecological problems, but also for insomnia, cold sensation of the lower extremities, and skin abnormalities, and thus the Korean Ministry of Food and Drug Safety recognized OGT extract to be used for “The following symptoms of patients with burning hands and feet and dry lips: menstrual irregularities, dysmenorrhea, leukorrhea, menopausal disorders, insomnia, nervousness, eczema, cold sensation in the lower extremities, and frostbites”7. However, until recently, the main evidence related to the effectiveness of OGT has been limited to gynecological areas such as menopausal disorders8,9 and female reproductive diseases10,11, and evidence related to its efficacy in neuropsychology such as insomnia and anxiety is lacking. With this background, a retrospective analysis was conducted in the medical records of cerebrovascular disease, Parkinson’s disease, and Alzheimer’s disease patients who took OGT extract as an insomnia treatment, one of the various indications for OGT.
As a result, improvement in insomnia symptoms was confirmed in 20 out of 24 patients (83.3%) after administration of the OGT extract, with a statistically significant decrease in the total PSQI-K score from 13.08±4.54 to 10.42±4.58 (p<0.001). This change was not only statistically significant, but also clinically significant because it showed an improvement close to 3 points, which is the MCID of PSQI-K15. In addition, adverse side effects from OGT extract administration were investigated based on patient complaints on medical records, and not a single adverse reaction report was confirmed due to OGT administration. Therefore, the results of this study suggest that OGT extract administration may be a relatively safe and effective therapeutic option for insomnia in patients with neurological diseases.
For the following reasons, the present study aimed to evaluate the effect of OGT on insomnia in patients with cerebrovascular disease or neurodegenerative diseases (for example, Parkinson disease, Parkinsonism, or Alzheimer’s disease). Cerebrovascular disease or neurodegenerative diseases usually occurs in the elderly over 65 years of age. Therefore, patients with these diseases are known to show many symptoms of “kidney deficiency” and are reported to have significant associations with “static blood”17-19. The efficacy of OGT is known as “warm the meridian to dissipate cold” and “tonify blood and dispel stasis”20. Based on these efficacies, OGT has been used for pathological conditions of “insecurity of the thoroughfare and conception vessels” and “static blood”20. For this reason, we assumed that OGT would be more suitable for the insomnia disorders of patients with cerebrovascular disease or neurodegenerative diseases than other herbal prescriptions that have been used for insomnia disorders.
In the present study, we compared the characteristics of responder of OGT and non-responder of OGT to clarify specific indications for OGT. As a result, although no indicator showing a significant difference between the two groups was identified, it was confirmed that the predicted probabilities of the two groups were contradictory in the Qi deficiency (negative probabilities in responder and positive probabilities in non-responder). We presume that these results reflect the pharmacological efficacy of OGT. As we mentioned above, the efficacy of OGT is “warm the meridian to dissipate cold” and “tonify blood and dispel stasis”20. Therefore, OGT could be an herbal prescription suitable for the condition of “blood” rather than “Qi”. Reflecting this efficacy of OGT, it seems that the probabilities of Qi deficiency were negative in OGT responders.
However, this study has the following limitations. First, due to the retrospective nature of the study design, the medical records of participants with insufficient information could not be analyzed; thus, the treatment effect of the OGT may have been overestimated. Second, electrolyte test and laboratory test findings before and after OGT administration were not studied, and the study relied on patient complaints to assess safety. Therefore, the safety information identified by this study is limited in its results. Third, PSQI-K is a questionnaire used to assess the sleep status in the last 4 weeks13, but in this study, due to the short observation period, the duration was adjusted to the last 2 days. Therefore, the confidence in the PSQI-K score evaluated in this study is also limited. Fourth, this review did not perform long-term follow-up of OGT administration. The observation period in this study was relatively short, with an average of 14.42±9.2 days, and no evaluation was conducted after the end of OGT administration. Therefore, the long-term effects of OGT medication on insomnia disorder in patients with neurological diseases could not be evaluated.
Despite these limitations, this study has the following implications: First, in addition to the improvement of subjective symptoms, PSQI-K, a reliable and valid questionnaire13, was used to evaluate the effect of OGT on insomnia more objectively. Second, by comparing the characteristics of OGT responders and non-responders for insomnia, the study was able to provide information that may be helpful when OGT is used for insomnia in clinical settings. Although we did not find any statistically significant differences between responders and non-responders, we found that the higher the probability of Qi-deficiency during pattern identification, the more likely that it does not correspond to the response from the OGT extract. Third, we identified the potential of OGT as a new treatment option for insomnia in patients with neurological diseases. Various hypnotics are usually used for insomnia. However, taking hypnotics is known to adversely affect the prognosis of patients with neurological diseases. Patients with cerebrovascular diseases are known to have an increased risk of falls when taking hypnotics compared to those who do not4, and one study suggested that hypnotics are one of the top three medications that most frequently cause drug-related problems in patients with cerebrovascular diseases21. In addition, since some hypnotics such as trazodone are known to cause Parkinsonism6, it has been suggested that hypnotics should be used with caution even in patients with Parkinson’s disease. Finally, in patients with Alzheimer’s disease, the use of benzodiazepine-based medications, one of the most used hypnotics, is known to accelerate cognitive decline and requires caution5. Therefore, patients with neurological disorders with insomnia need new drug therapies that do not have sedative effects such as hypnotics, and OGT may be a new alternative.
V. Conclusion
However, despite the significant results, due to the limitations of the study mentioned above, OGT extract can only be confirmed as a new potential treatment option of insomnia in patients with neurological diseases but has not provided any conclusive evidence. In the future, prospective case-based or prospective placebo-controlled, randomized, double-blind trials are needed to overcome these limitations and provide more conclusive evidence.